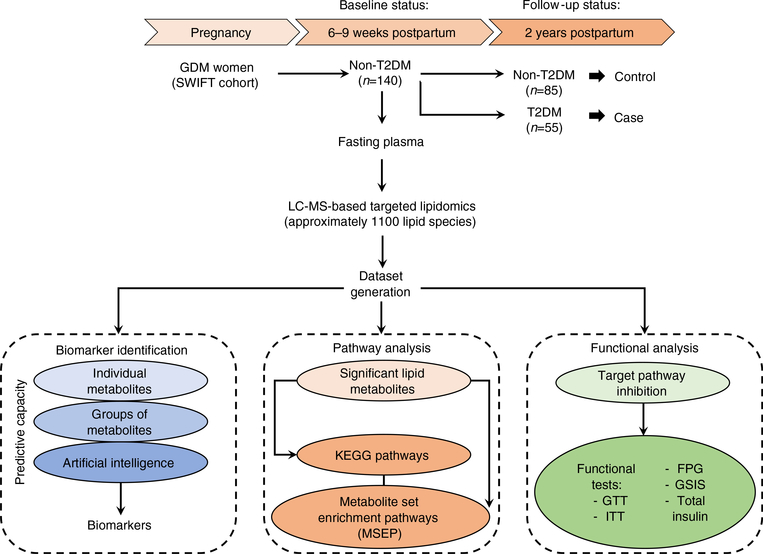
Fig. 1.
The schematic flow diagram of the study design. This was a nested case–control study within the SWIFT study, a prospective cohort of 1035 women diagnosed with GDM and followed up to 2 years postpartum. A total of 140 women were selected out of the 1035 SWIFT participants. These women did not have type 2 diabetesmellitus (T2DM) at 6–9 weeks postpartum(study baseline) based on 2 h 75 g OGTT. Of the 140 selected, 55 women were diagnosed as having T2DM, via 2 h 75 g OGTTs, within 2 years post baseline. This group was termed as ‘case’. The remaining 85 women did not develop T2DMbased on the results of the 2 h 75 g OGTTs within 2 years post baseline. This group was termed ‘control’ (non-T2DM). The fasting plasma from the baseline examination was used for LC-MS-based targeted lipidomics aimed at finding the relation in terms of a predictive signature and the earlier stage pathophysiology of T2DM prospectively within the 2 year follow-up period