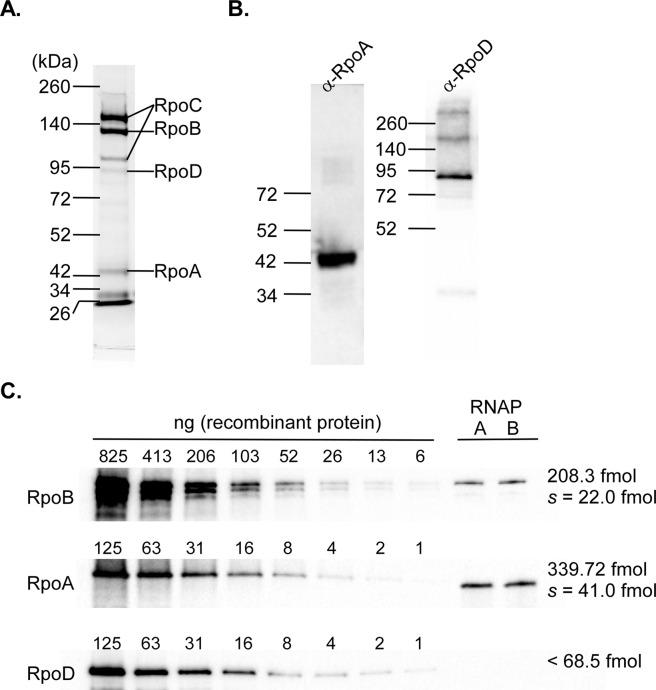
Figure 2.
Purification of the RNA polymerase from B. burgdorferi and determination of the molar ratio of the core subunits β and α. (A) Purified proteins in pooled elution fraction from nickel-affinity chromatography performed on lysates generated from B. burgdorferi 5A4-RpoC-His10X were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Labels on the right side of the gel indicate RNA polymerase subunits detected by LC-MS of excised bands. (B) Western blots were performed on nickel-affinity purified proteins using anti-Borrelia-RpoA and anti-Borrelia-RpoD antibodies to confirm the presence of the target proteins. Numbers indicate the migration of protein molecular mass markers. Detection of RpoD required loading microgram quantities of purified RNA polymerase. (C) Molar ratios were determined by quantitative western blots. Recombinant RpoB, RpoA, and RpoD were loaded in amounts indicated above to form a standard curve. Purified RNA polymerase samples A and B were loaded with the standard curve for quantification. Molar amounts were calculated from the theoretical masses of proteins based on amino acid sequence. Images are representative of four replicate experiments.