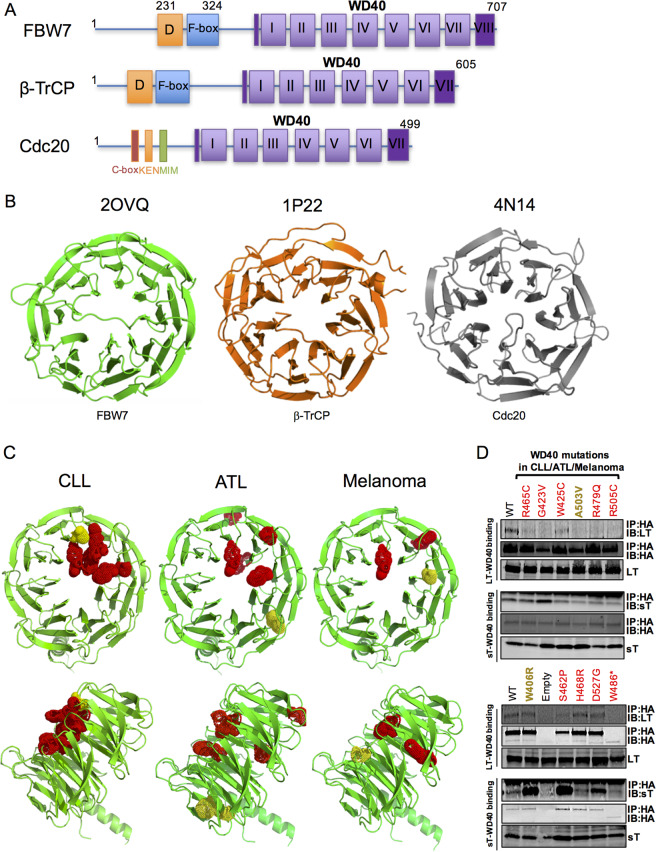
Fig. 1. Merkel cell polyomavirus small T targets WD40, a common domain structure of E3 ligases.
a Schematic organization of FBW7, β-TrCP, and Cdc20 genes. Diagram compares substrate-binding regions of SCF and Anaphase-Promoting Complex/Cyclosome (APC/C): FBW7, β-TrCP, and Cdc20. Color-coded exons corresponding to each specific domain: WD40 repeats mediate substrate recognition (purple); F-box recruits the SCF complex (blue); dimerization domain (orange). Cdc20 has unique domains such as C box, KEN box, Mad2-interacting motif (MIM). A common feature among these E3 ligases is the WD domain. The first β-strand of WD40 completes the last blade structure (dark purple) to create the closed ring propeller-structure. b Classical tertiary structure of the WD40 domains of E3 ligases. Ribbon diagrams of WD40 repeat domains of human FBW7, β-TrCP and Cdc20 and their respective PDB ID numbers. c Ribbon diagram of FBW7 WD40 domain mutations identified in CLL, ATL and melanoma. β-propeller blades are highlighted in green (top-down and side views). Localization of FBW7 WD40 mutations identified in CLL, ATL and melanoma human cancers are highlighted in red and yellow spheres30–32. Mutations contributing to either transforming activity or reducing substrate binding/degradation are depicted as red spheres and mutations without a loss of function are illustrated in yellow spheres. CLL-associated FBW7 mutations are located on a ‘hotspot', the top face of WD40 domain5, affecting binding of its substrates to the WD40. In contrast, mutations in ATL and melanoma are less likely to be spatially clustered towards the top face, but instead are buried. d WD40 domain mutations in human cancers affect LT, but not sT, binding. Mutations identified in CLL, ATL and melanoma were introduced. The interaction between sT or LT and FBW7 were analyzed by co-immunoprecipitation analysis in 293 cells transfected with LT or sT and HA-tagged WT WD40 (FBW7ΔDF) or mutants. The asterisk (*) indicates a nonsense mutation.