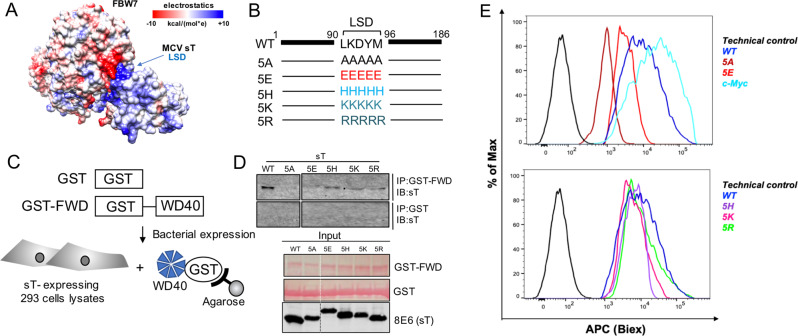
Fig. 4. MCV sT LSD is positively charged and required for sT oncogenic function.
a Electrostatic surface view of MCV sT and WD40 domain structures. The surface electrostatic potentials of WD40 and MCV sT are shown. Positive electrostatic potential is denoted in blue and negative potential in red. LSD residues (91–95) elicit an overall positive electrostatic charge due to the presence of charged amino acids. b Mutations in LSD. The LSD amino acids LKDYM (residues 91–95) were mutated to neutral (A), negatively charged (E), weak positively charged (H) and strongly positively charged (K, R) residues to test the effects of surface charge in WD40 interaction and substrate stabilization. c GST pulldown assay. The immobilized bait protein (GST or GST-FBW7 WD40 (GST-FWD)) purified from E. coli was incubated with 293 cell lysates expressing wild-type or mutant sT. d Positive charge of LSD is critical for the binding to the WD40 domain. Both neutral (5A) and acidic amino acids (5E) mutations in LSD lost its binding whereas basic amino acids mutation in the LSD restored its binding to the WD40. GST proteins (GST and GST-FWD) were purified and visualized on the membrane using Ponceau S. The bait protein used in each pulldown is >250 ng as detected by Ponceau S (Sigma). 2% of each prey sample was loaded for sT expression detection, using 8E6 antibody. e Quantitative analysis of wild-type and LSD mutant sT interactions with the FBW7 using a proximity ligation assay-flow cytometry analysis. A PLA-flow cytometric analysis was performed to further validate the quantitative interaction of FBW7 and LSD mutants. Primary antibodies were utilized at optimized concentrations with HA-Tag (C29F4) rabbit mAb (1:500), c-Myc (9E10) mouse mAb (1:500), and 2T2 (1:500) (Millipore). Protein expression was evaluated by immunoblot analysis in Supplementary Fig. S5C.