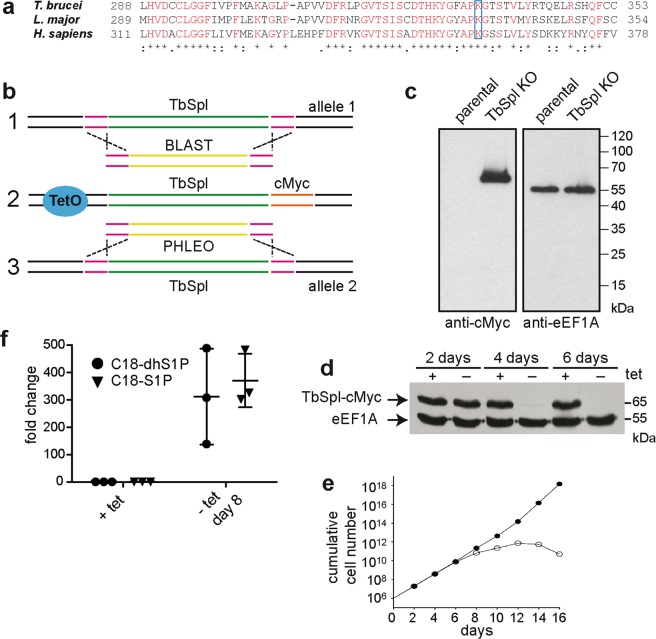
Figure 1.
Generation of inducible TbSpl-knockout cells. (a) Partial sequence alignment of T. brucei, L. major and H. sapiens sphingosine-1-phosphate lyase sequences. Conserved amino acids are shown in red, the boxed lysine residues show the conserved catalytic site. (b) Strategy to generate conditional knockout parasites. First, the ORF of TbSpl from one allele was replaced with a blasticidin (BLAST) resistance gene (1). After antibiotic selection and PCR confirmation of correct replacement, a tetracycline-inducible c-myc-tagged ectopic copy of TbSpl was inserted into the genome (2), then the second allele of TbSpl was replaced with a phleomycin (PHLEO) resistance gene (3). (c) Immunoblot analysis of whole cell lysates from parental cells and TbSpl KO parasites expressing TbSpl-cMyc. eEF1A was used as a loading control. (d) Immunoblot analysis of whole cell lysates. Cells were cultured in the presence (+) or absence (−) of tetracycline for 2, 4 or 6 days and proteins detected by Western blotting. The positions of TbSpl-cMyc and eEF1A are indicated. (e) Growth of conditional TbSpl KO cells cultured in the presence (•) or absence (o) of tetracycline. (f) Relative quantification of cellular S1P levels from TbSpl KO cells cultured in presence (+) or absence (−) of TbSpl expression for 8 days. Levels of C18-S1P and C18-dhS1P (dihydrosphingosine-1-phosphate) were quantified. Individual data points (+/−SD) of fold increases of three independent determinations are shown.