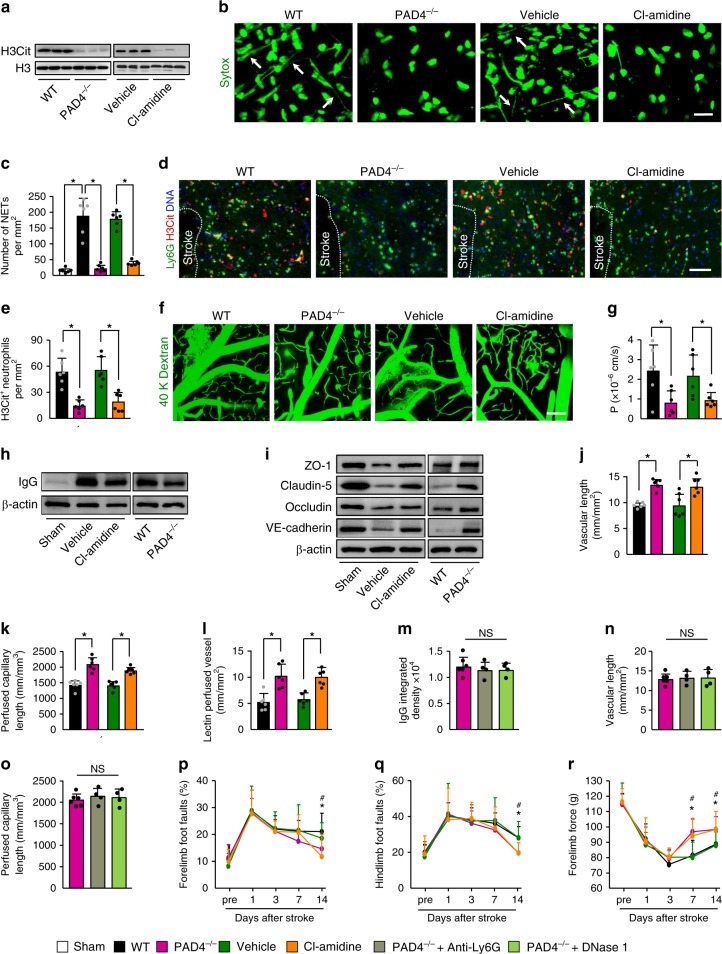
Fig. 7. PAD4 deficiency or pharmacologic inhibition promotes vascular remodeling after stroke.
a Representative immunoblots of H3Cit levels in the peri-infarct cortex at 3 days in WT and PAD4−/− mice, and WT mice treated with vehicle or the PAD inhibitor Cl-amidine. Independent experiments are repeated at least three times. b In-vivo multiphoton microscopic images of extracellular DNA (Sytox, green) in the peri-infarct cortex of mice at 3 days after stroke. Arrows indicate extracellular DNA fibers. Bar = 20 µm. Independent experiments are repeated at least three times. c Quantification of NETs for each group (n = 6). One-way ANOVA test was applied with *P < 0.0001 (Same vs. WT stroke), *P < 0.0001 (WT vs. PAD4−/−), *P < 0.0001 (Vehicle vs. Cl-amidine). d Representative confocal images of neutrophil (Ly6G, green) and H3Cit (red) immunostaining in the peri-infarct cortex at 3 days. DNA was stained with Hoechst 33342 (blue). Bar = 40 μm. e Quantification of the numbers of H3Cit-positive neutrophils in the peri-infarct cortex at 3 days in WT and PAD4−/− mice, and WT mice treated with vehicle or the PAD inhibitor Cl-amidine (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0002 (WT vs. PAD4−/−), *P = 0.0010 (Vehicle vs. Cl-amidine). f, g In-vivo multiphoton microscopic images (f) of intravenously injected FITC-dextran leakage in cortical vessels at 14 days and quantification of the permeability (P) product of FITC-dextran (g) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0197 (WT vs. PAD4−/−), *P = 0.0260 (Vehicle vs. Cl-amidine). Bar = 100 μm. h Representative immunoblots of IgG levels in capillary-depletion brain tissue at 14 days in WT and PAD4−/− mice, and WT mice treated with vehicle or Cl-amidine. Independent experiments are repeated at least three times. i Representative immunoblots of the tight-junction protein ZO-1, claudin-5, and occludin, and the adherens junction protein VE-cadherin in isolated brain microvessels at 14 days. Independent experiments are repeated at least three times. j–l Quantification of microvascular density (j), perfused capillary length (k), and tomato-lectin perfused vessels (l) in the peri-infarct cortex at 14 days (n = 6). Unpaired two-tailed Student’s t-test was applied with *P < 0.0001 (WT vs. PAD4−/− (j)), *P = 0.0093 (Vehicle vs. Cl-amidine (j)), *P = 0.0001 (WT vs. PAD4−/− (k)), *P < 0.0001 (Vehicle vs. Cl-amidine (k)) *P = 0.0015 (WT vs PAD4−/− (l)), *P = 0.0013 (Vehicle vs. Cl-amidine (l)). m–o Quantification of extravascular IgG deposits (m), microvascular length (n), and perfused capillary length (o) in the peri-infarct cortex at 14 days after stroke (n = 6 for PAD4−/−, n = 4 for PAD4−/− + Anti-Ly6G and PAD4−/− + DNase 1). One-way ANOVA test was applied with P = 0.7263 (m), P = 0.9547 (n), P = 0.7304 (o). p–r PAD4 deficiency or Cl-amidine treatment improved neurological functions in beam walking test (p, q) and forelimb force test (r) (n = 10). One-way ANOVA test was applied with *P = 0.0407 (p), *P = 0.0056 (q), *P = 0.0013 (7d (r)) and *P = 0.0172 (14d (r)) (PAD4−/− and WT), #P = 0.0439 (p) and #P = 0.0210 (q), #P = 0.0091 (7d (r)) and #P = 0.0394 (14d (r)) (Cl-amidine and vehicle). Data are presented as mean ± SD. Source data underlying graph a, c, e, and g–r are provided as a Source Data file.