FIGURE 2.
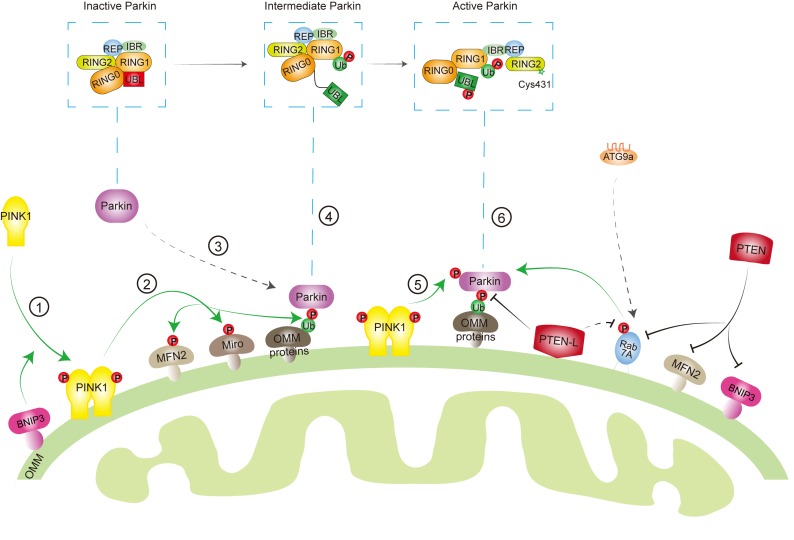
Key effectors involved in mitophagy machinery. When mitochondria are healthy, phosphatase and tensin homolog-induced kinase protein 1 (PINK1) is imported into the mitochondria, cleaved by protease, and degraded by proteasome, while Parkin keeps in an inactive conformation in the cytosol through intradomain–domain interactions. Upon mitochondrial damage or depolarization, PINK1 is stabilized and activated at the outer mitochondrial membrane (OMM) ➀, which leads to the phosphorylation of its downstream targets, such as ubiquitin (Ub) ➁. Parkin has a high affinity to phosphorylated Ub (pSer65-Ub), which recruits Parkin from cytosol to mitochondria ➂. Several other factors, such as mitofusin 2 (MFN2), Miro, Rab7A, as well as BCL2/adenovirus E1B 19 kDa protein-interacting proteins 3 (BNIP3) are also involved in Parkin mitochondrial recruitment. Binding to pSer65-Ub releases the Ub-like (UBL) domain of Parkin from RING1 domain, partially activating Parkin ➃. Then, PINK1 phosphorylates the UBL domain at Ser65 ➄, which drives the phospho-UBL to rebind to the RING0 domain of Parkin to expose RING2′ catalytic site (Cys431) and fully activate Parkin ➅. On the other hand, phosphatase and tensin homolog long (PTEN-L) located at OMM dephosphorylates Ub to inhibit mitophagy, whereas PTEN in the cytosol suppresses mitophagy through targeting Rab7A, MFN2, or BNIP3.