Abstract
Background
Few data are reported in the literature about the outcome of patients with severe extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) infections treated with ceftolozane/tazobactam (C/T), in empiric or definitive therapy.
Methods
A multicenter retrospective study was performed in Italy (June 2016–June 2019). Successful clinical outcome was defined as complete resolution of clinical signs/symptoms related to ESBL-E infection and lack of microbiological evidence of infection. The primary end point was to identify predictors of clinical failure of C/T therapy.
Results
C/T treatment was documented in 153 patients: pneumonia was the most common diagnosis (n = 46, 30%), followed by 34 cases of complicated urinary tract infections (22.2%). Septic shock was observed in 42 (27.5%) patients. C/T was used as empiric therapy in 46 (30%) patients and as monotherapy in 127 (83%) patients. Favorable clinical outcome was observed in 128 (83.7%) patients; 25 patients were considered to have failed C/T therapy. Overall, 30-day mortality was reported for 15 (9.8%) patients. At multivariate analysis, Charlson comorbidity index >4 (odds ratio [OR], 2.3; 95% confidence interval [CI], 1.9–3.5; P = .02), septic shock (OR, 6.2; 95% CI, 3.8–7.9; P < .001), and continuous renal replacement therapy (OR, 3.1; 95% CI, 1.9–5.3; P = .001) were independently associated with clinical failure, whereas empiric therapy displaying in vitro activity (OR, 0.12; 95% CI, 0.01–0.34; P < .001) and adequate source control of infection (OR, 0.42; 95% CI, 0.14–0.55; P < .001) were associated with clinical success.
Conclusions
Data show that C/T could be a valid option in empiric and/or targeted therapy in patients with severe infections caused by ESBL-producing Enterobacterales. Clinicians should be aware of the risk of clinical failure with standard-dose C/T therapy in septic patients receiving CRRT.
Keywords: ceftolozane/tazobactam, CRRT, Enterobacterales, ESBL, septic shock
The incidence of severe infections caused by extended-spectrum β-lactamase (ESBL)–producing Enterobacterales (E) is a rising concern worldwide, owing to the successful dissemination of these species in both the community and health care–associated ecosystem [1–3]. This situation has led to a dramatic increase in the use of carbapenems in high-prevalence countries, which is suspected to contribute to the ongoing pandemic of carbapenemase-producing Enterobacterales. Therefore, alternative treatment options and carbapenem-sparing regimens for patients with serious infections caused by ESBL-E are urgently needed [4].
Ceftolozane/tazobactam (C/T) is a novel β-lactam/β-lactamase inhibitor (BLBLI) combination that has shown potent activity against gram-negative bacteria [5]. It is the combination of a novel cephalosporin, structurally like ceftazidime, with a well-known β-lactamase inhibitor. Similar to ceftazidime and other expanded-spectrum cephalosporins, ceftolozane is not stable when combined with ESBL. For this reason, a formulation for clinical use has been developed in combination with tazobactam, a mechanism-based β-lactamase inhibitor that extends the activity of ceftolozane against many ESBL-E [6].
Pivotal clinical trials of C/T have demonstrated its efficacy and safety for the treatment of ESBL infections [7, 8] and clinical experiences with C/T are accumulating and expanding. However, these studies are almost completely focused on treatment of Pseudomonas aeruginosa infections [9–11]. Information regarding its real-life use, efficacy, and tolerability against ESBL-E in daily clinical practice is limited. Here we report a clinical multicenter experience with C/T to treat serious infections due to ESBL-E since its approval in Italy.
METHODS
Study Setting and Design
This was a multicenter retrospective study in which we included all adult patients treated with C/T (for at least 96 hours) for any confirmed infection produced by ESBL-producing Enterobacterales between June 2016 and June 2019 (36-month period) in 12 hospitals in Italy. The Internal Review Board of Medical Area (DAME) of the coordinating center (Azienda Ospedaliera Universitaria Integrata di Udine, Udine, Italy) approved this study. Because of its retrospective nature, informed consent was considered unnecessary.
Cases were eligible for the cohort study if the patient (i) was aged ≥18 years, (ii) received ≥96 hours of C/T (with or without other antibiotics), and (iii) had a culture-confirmed ESBL-E infection.
Data Collection
Patients’ medical records were retrospectively reviewed, and data were collected using a pre-established form. The following data were recorded: age and sex, underlying diseases according to Charlson comorbidity index, type of infection, presence of sepsis or septic shock at the time of the infection, susceptibility pattern of ESBL-E isolates, date of start and end of C/T therapy, source control of infection, when applicable, other antibiotics administered before, concomitant to, and after C/T therapy, reasons for C/T use, dosage(s) of C/T and length of therapy, adverse events (AEs), clinical outcome, and recurrence of infection.
Definitions
Chronic renal disease was defined as the need for hemodialysis or the presence of renal impairment (serum creatinine >1.5 mg/dL) at the time of hospital admission. Diagnosis and classification of infections were defined according to the criteria of the US Centers for Disease Control and Prevention (CDC) [12]. Sepsis and septic shock were defined according to standard international criteria [13]. Source control of infection was considered adequate when any additional measures were taken to control the focus of the infection in the 48 hours after infection onset (ie, removal of urinary catheter or intravascular catheter, as well as surgical or radiological drainage of collection).
An infection was considered “life-threatening” when a patient (i) received C/T as rescue therapy because of clinical failure of a previous antibiotic regimen, (ii) had septic shock at the time of ESBL-E infection, or (iii) required intensive care unit (ICU) admission at the time of ESBL-E infection. Recurrence was considered to have occurred if the infection reappeared after antibiotic discontinuation.
Antibiotic Therapy
Indications for C/T and dosage, type of infusion, and duration were established by infectious diseases specialists in each participating center, based on knowledge of previous colonization, clinical presentation, and local guidelines. C/T was dosed either as approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as an intravenous (i.v.) dose of 1.5 g every 8 h (q8h; standard dosage) or as supported by recent data in patients with nosocomial pneumonia [14] as an i.v. dose of 3 g q8h (off-label dosage at time of treatment); moreover, dosages were adjusted according to creatinine clearance. Dose adjustment was required only for patients with moderate renal dysfunction (creatinine clearance < 50 mL/min). In patients receiving continuous renal replacement therapy (CRRT), C/T was administered at 1.5 g q8h, as suggested by pharmacokinetic studies [15, 16]. AEs were classified according to World Health Organization (WHO) definitions [17].
Depending on the number of drugs used, treatment regimens were classified either as monotherapy or combination therapy. Initial antibiotic therapy, defined as empirical antimicrobial chemotherapy implemented within 24 hours after the onset of infection, was assessed, along with definitive antibiotic therapy, defined as antimicrobial treatment based on in vitro ESBL-E isolate susceptibilities. Drugs in definitive therapy must have been administered for at least 50% of the total duration of therapy (except for patients who died while on definitive therapy, who were included if they received at least 1 complete day of therapy). Time to initial definitive therapy was the period between the infection onset and initial definitive therapy.
Definition of Patient Outcome
Patient outcome was assessed as success or failure at the end of the follow-up period, which finished at the end of August 2019. A successful clinical outcome was defined as complete resolution of clinical signs and symptoms related to ESBL-E infection and lack of microbiological evidence of infection. Clinical failure was defined as either lack of clinical response and/or recurrence and/or attributable mortality due to ESBL-E infection. Specifically, clinical failure was defined as a composite of the following: (i) 30-day mortality; (ii) ongoing fever after 5 days of therapy; (iii) persistence of leukocytosis after 5 days of therapy; (iv) presence, after 5 days of therapy, of clinical signs of infection that could not be attributed to causes other than ESBL-E infection. Clinical success was defined also as absence of clinical failure.
Microbiological Methods
All ESBL-E isolates were processed at each participating center according to their own practice. In all cases, antibiotic susceptibility was determined by the Vitek 2 (Biomerieux, France) automatic method. ESBL-producing strains were phenotypically identified from baseline specimens using the following criteria: minimum inhibitory concentration (MIC) >1 mg/L for a third-generation cephalosporin (ceftazidime and/or cefotaxime or ceftriaxone); phenotypic ESBL confirmation for Enterobacterales in which chromosomal AmpC is uncommon (E. coli, Klebsiella spp., Proteus spp.) with an MIC decrease of >3 dilutions when combined with clavulanic acid; phenotypic ESBL confirmation for Enterobacterales in which chromosomal AmpC β-lactamase is common (Enterobacter spp., Citrobacter spp., Serratia spp., Providencia spp.) using an MIC of cefepime with a decrease of >3 dilutions when cefepime is combined with clavulanic acid. However, ESBL detection in each center was confirmed as previously reported [18].
Susceptibility of bacteria to C/T was determined by the Etest (Liofilmchem, Roseto degli Abruzzi, Italy), and the results were interpreted according to the breakpoints proposed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [19].
Statistical Analysis
The primary end point was to identify predictors of clinical failure of C/T therapy.
Continuous variables were compared using the Student t test and Mann-Whitney U test for normally and non–normally distributed variables, respectively. The χ 2 test or Fisher exact test was used to compare categorical variables. All pretreatment variables identified during univariate analysis were tested using logistic regression analysis to identify risk factors associated with clinical failure. In a multivariate analysis, the model was tested using a backward stepwise selection and P < .05 for all variables in order to determine the effects of all anamnestic, clinical, and therapeutic variables on clinical success or failure of C/T therapy. Empiric and definitive therapy were adjusted for confounders (definitive and empiric regimens, respectively). All tests of statistical significance were 2-tailed. Differences were considered statistically significant at a P value of <.05. Statistical analysis was performed with the software package PASW Statistics, version 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 153 patients were included during the study period, with a median number (interquartile range [IQR]) of 10 (2–28) enrolled cases among 12 centers. The baseline characteristics of study population are shown in Table 1. Overall, the median age (IQR) was 69 (48–77) years, 82 (53.6%) patients were male, and the mean ± SD Charlson comorbidity index score was 4.9 ± 3.6. The most frequent source of infection was hospital-acquired pneumonia (HAP) in 46 patients (30%, including 20 patients with ventilator-associated pneumonia), followed by complicated urinary tract infections (cUTIs; 22.2% of cases, 34/153), acute bacterial skin and skin structure infections (ABSSSIs; 16.3%), and complicated intra-abdominal infections (cIAIs; 16.3%, 25/153 each). Concomitant bloodstream infection was confirmed in 30.7% (47/153 patients).
Table 1.
Baseline Demographics and Clinical Characteristics of 153 Patients Included in the Efficacy Population Analysis
| Variable | n = 153 (%)a |
|---|---|
| Age, median (IQR), y | 69 (48–77) |
| Male sex | 82 (53.6) |
| Community-acquired infection | 10 (6.5) |
| Hospital-acquired infection | 143 (93.5) |
| Ward | |
| Medical | 98 (64) |
| Surgical | 25 (16.4) |
| ICU | 30 (19.6) |
| Charlson comorbidity index, mean ± SD | 4.9 ± 3.6 |
| Underlying diseases | |
| Cardiac disease | 56 (36.6) |
| Neurological disease | 53 (34.6) |
| Chronic renal disease | 51 (33.3) |
| Diabetes mellitus | 42 (27.4) |
| Gastrointestinal disease | 41 (26.7) |
| Solid-organ tumor | 37 (24.1) |
| Solid-organ transplant | 19 (12.4) |
| Hematological malignancy | 20 (13) |
| COPD | 35 (22.8) |
| Liver disease | 22 (14.3) |
| Other predisposing conditionsb | |
| Corticosteroids | 52 (33.9) |
| Other immunosuppressive therapy | 29 (18.9) |
| Chemotherapy | 17 (11.1) |
| Neutropeniac | 15 (9.8) |
| Invasive procedures | |
| Central venous catheter | 76 (49.7) |
| Urinary catheter | 111 (72.5) |
| Previous surgeryb | 58 (37.9) |
| Mechanical ventilation/NIV | 28 (18.3) |
| Percutaneous endoscopic gastrostomy | 2 (1.3) |
| Intermittent hemodialysis | 25 (16.3) |
| CRRT | 18 (11.7) |
| Previous ESBL-E colonizationb | 50 (32.6) |
| Severity of clinical presentation | |
| No sepsis | 52 (33.9) |
| Sepsis | 59 (38.6) |
| Septic shock | 42 (27.5) |
| ICU admission due to ESBL-E infection | 74 (48.3) |
| Type of infection | |
| Nosocomial pneumoniae | 46 (30) |
| ABSSSI | 25 (16.3) |
| cUTI | 34 (22.2) |
| cIAI | 25 (16.3) |
| Bone infection | 5 (3.2) |
| Primary bacteremia | 16 (10.4) |
| Other infectionsd | 2 (1.3) |
| Concomitant ESBL-E bacteremia | 47 (30.7) |
| Life-threatening infection | 91 (59.4) |
| Polymicrobial infection | 31 (20.2) |
| Antibiotics before C/T treatment | |
| Received antibiotics before C/T for current infection | 52 (33.9) |
| No. of antibiotics received, median (range) | 1 (1–3) |
| Days of antibiotic therapy, median (range) | 6 (3–14) |
| C/T treatment | |
| Empiric treatment | 46 (30) |
| Combination therapy | 26 (16.9) |
| Time from infection onset to C/T administration, median (IQR), d | 6 (3–15) |
| Days of treatment, median (range) | 14 (8–25) |
| Extended infusion | 34 (22.2) |
| Continuous infusion | 11 (7.2) |
| Intermittent infusion | 108 (70.6) |
| Standard dosage (or adjusted according to creatinine clearance)f | 115 (75) |
| Off-label dosage | 38 (25) |
| Adequate source control of infection | 47/57 (82.4) |
| Successful clinical outcome | 128 (83.7) |
| 30-d mortality | 15 (9.8) |
Abbreviations: ABSSSI, acute bacterial skin and skin-structure infection; C/T, ceftolozane/tazobactam; cIAI, complicated intra-abdominal infection; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; cUTI, complicated urinary tract infection; ICU, intensive care unit; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation.
aData are presented as No. (%) unless otherwise stated.
bWithin the previous 30 days.
cAbsolute neutrophil count <500/mm3.
dOther infections include central venous catheter–related bacteremia (n = 1) and community-acquired pneumonia (n = 1).
eThirty-two patients with hospital-acquired pneumonia and 14 with ventilator-associated pneumonia.
fSix patients with augmented renal clearance.
The etiology of infection and antimicrobial susceptibility pattern of ESBL-E isolates are reported in Table 2. Escherichia coli was the most frequently isolated pathogen (48.3%), followed by Klebsiella pneumoniae (29.4%) and Enterobacter spp. (14.4%).
Table 2.
Etiology of Infection and Antimicrobial Susceptibility Pattern of ESBL-Producing Enterobacterales Isolates
| Etiology | n = 153 (%) |
|---|---|
| Escherichia coli | 74 (48.3) |
| Klebsiella pneumoniae | 45 (29.4) |
| Enterobacter spp. | 22 (14.4) |
| Serratia marcescens | 5 (3.3) |
| Proteus spp. | 3 (2) |
| Citrobacter spp. | 2 (1.3) |
| Morganella morganii | 2 (1.3) |
| Antimicrobial Agent | No. of Susceptible Strains (%) |
| Amikacin | 128 (83.6) |
| Ceftolozane/tazobactam | 153 (100) |
| Ciprofloxacin | 74 (48.3) |
| Colistin | 145 (94.7) |
| Gentamicin | 119 (77.7) |
| Fosfomycin | 132 (86.2) |
| Levofloxacin | 88 (57.5) |
| Meropenem | 153 (100) |
| Piperacillin/tazobactam | 104 (67.9) |
| Imipenem/cilastatin | 153 (100) |
Abbreviation: ESBL, extended-spectrum β-lactamase.
At the time of infection, 42 (27.5%) patients presented with septic shock and 91 (59.4%) patients were classified as having a life-threating infection. Overall, source control of infection was considered necessary in 57 patients (37.2%), and in 47/57 cases (82.4%) it was considered adequate.
In most patients, C/T was administered once in vitro susceptibility was confirmed (70%, 107/153), and the median time from infection onset to C/T administration (IQR) was 6 (3–15) days; C/T doses were 1.5 g q8h (or adjusted according to creatinine clearance) in 115 patients (75%) and 3 g q8h in 38 patients (25%). Eighty-three percent of the patients received C/T as monotherapy in definitive therapy; in 26 (16.9%) patients, it was used as combination definitive therapy. No statistically significant differences were reported in the use of C/T in mono or combo definitive therapy with regards to clinical failure (P = .34). Overall, C/T was administered for a median duration (IQR) of 14 (8–25) days.
When used as second-line or later, the most common reasons for discontinuation of previous antibiotics were in vitro resistance of strains (69.1%) and clinical failure of previous therapy (30.9%). Piperacillin-tazobactam was the first-line therapy in 35% of patients, followed by cephalosporins (44%) and quinolones (12%). The median follow-up period of the study population (IQR) was 19 (6–49) days.
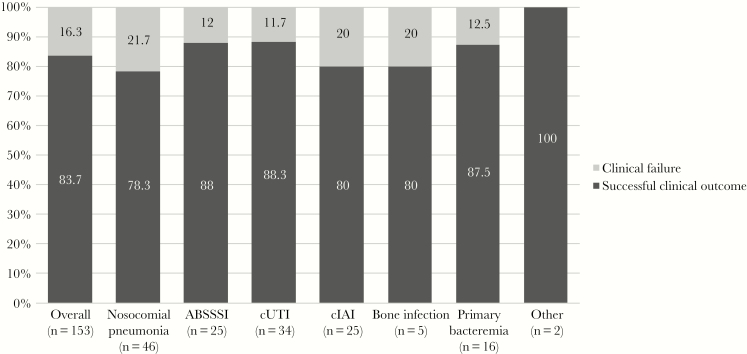
Overall, 128 (83.6%) patients experienced a successful clinical outcome. Clinical failure was reported in 25 patients: Lack of clinical response was recorded in 4 (16%) patients, recurrence of ESBL-E infection in 6 (24%), and attributable mortality in 15 (60%). Figure 1 stratifies the clinical outcome of patients treated with C/T according to the site of infections. Clinical success was observed in 88.3% of patients with cUTI, 88% with ABSSSI, 87.5% with primary bacteremia, 80% with cIAI and bone infections, and 78.3% with hospital-acquired pneumonia. Among 54 (35.2%) patients treated with piperacillin-tazobactam as first-line therapy, clinical failure was reported in 11 patients: 5 patients died, and 6 patients showed lack of clinical response. According to etiology, a successful clinical outcome was reported in 67/74 (90.5%) cases of Escherichia coli, 38/45 (84.4%) of Klebsiella pneumoniae, and 15/22 (68.2%) of Enterobacter spp. infections. All baseline strains showed susceptibility to C/T and carbapenems, whereas only 104 (67.9%) showed susceptibility to piperacillin/tazobactam. Despite this, C/T resistance developed during C/T therapy in 3 patients (1.9%), none of whom had a fatal outcome: 1 patient with HAP (duration of C/T therapy 14 days), 1 patient with primary bacteremia (duration of C/T therapy 15 days), and 1 patient with cIAI (duration of C/T therapy 20 days). All these patients were treated with a standard C/T dosage, with etiology of infection due to Klebsiella pneumoniae. The MIC value pretreatment with C/T was ≤1 µg/mL; postexposure it was >32 µg/mL in 2 patients and >8 µg/mL in 1 patient. Of 6 (3.9%) patients with augmented renal clearance, clinical failure was recorded in 2 cases (33.3%).
Figure 1.
Comparison of successful clinical outcome in patients receiving ceftolozane/tazobactam in different sites of infection. Abbreviations: ABSSSI, acute bacterial skin and skin-structure infection; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection.
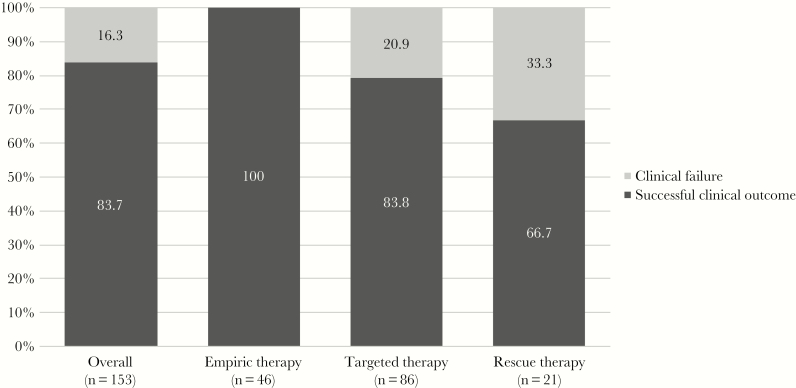
Comparison of successful clinical outcome in patients receiving C/T as empiric therapy in comparison with those who received C/T as targeted or rescue therapy is reported in Figure 2. Clinical success was reported in 100% (46/46 cases) of patients treated with empiric therapy, compared with 83.8% (68/86 cases) of targeted therapy and 66.7% (14/21 cases) of rescue therapy (P < .0001) cases.
Figure 2.
Comparison of successful clinical outcome in patients receiving ceftolozane/tazobactam (C/T) as empiric therapy in comparison with those who received C/T as targeted or rescue therapy.
In Table 3 is reported univariate analysis of risk factors associated with clinical failure of C/T therapy. ICU hospitalization at the time of infection (40% vs 15.6%; P = .01), higher mean Charlson comorbidity index (5.4 points vs 3.6 points; P < .001), chronic renal disease (60% vs 28.1%; P < .001), need for mechanical ventilation or noninvasive ventilation (40% vs 14%; P = .004), CRRT (40% vs 6.2%; P < .001), and septic shock (80% vs 17.2%; P < .001) were associated with clinical failure of C/T therapy. Conversely, receipt of C/T as empiric therapy (35.9% vs 0%; P < .001) and adequate source control of infection (75.4% vs 7.1%; P < .001) were associated with clinical success of C/T therapy. A lower time from infection onset to C/T administration was reported in patients with clinical success (4 vs 7 days; P < .001). Table 4 shows descriptions of patients who experienced clinical failure with C/T therapy.
Table 3.
Univariate Analysis of Risk Factors for Clinical Failure of C/T Therapy Among Patients With Enterobacterales Infection
| Variable | Clinical Success (n = 128), No. (%)a | Clinical Failure (n = 25), No. (%)a | P Value |
|---|---|---|---|
| Age, median (IQR), y | 69 (48–78) | 68 (47–77) | .92 |
| Male sex | 69 (53.9) | 13 (52) | 1.0 |
| Community-acquired infection | 9 (7) | 1 (4) | .78 |
| Hospital-acquired infection | 119 (92.9) | 24 (96) | .89 |
| Ward | |||
| Medical | 85 (66.4) | 13 (52) | .17 |
| Surgical | 23 (17.9) | 2 (8) | .37 |
| ICU | 20 (15.6) | 10 (40) | .01 |
| Charlson comorbidity index, mean ± SD | 3.6 ± 3 | 5.4 ± 2.6 | <.001 |
| Underlying diseases | |||
| Cardiac disease | 46 (35.9) | 10 (40) | .82 |
| Neurological disease | 46 (35.9) | 7 (28) | .49 |
| Chronic renal disease | 36 (28.1) | 15 (60) | <.001 |
| Diabetes mellitus | 35 (27.3) | 7 (28) | 1.0 |
| Gastrointestinal disease | 35 (27.3) | 6 (24) | .8 |
| Solid-organ tumor | 28 (21.8) | 9 (36) | .19 |
| Solid-organ transplant | 14 (10.9) | 5 (20) | .2 |
| Hematological malignancy | 15 (11.7) | 5 (20) | .28 |
| COPD | 29 (22.6) | 6 (24) | 1.0 |
| Liver disease | 18 (14) | 4 (16) | .92 |
| Other predisposing conditionsb | |||
| Corticosteroids | 45 (35.1) | 7 (28) | .64 |
| Other immunosuppressive therapy | 23 (17.9) | 6 (24) | .57 |
| Chemotherapy | 14 (10.9) | 3 (12) | 1.0 |
| Neutropeniac | 14 (10.9) | 1 (4) | .46 |
| Invasive procedures | |||
| Central venous catheter | 58 (45.3) | 18 (72) | .01 |
| Urinary catheter | 91 (71.1) | 20 (80) | .46 |
| Previous surgeryb | 48 (37.5) | 10 (40) | .82 |
| Mechanical ventilation/NIV | 18 (14) | 10 (40) | .004 |
| Percutaneous endoscopic gastrostomy | 2 (1.5) | 0 | 1.0 |
| Intermittent hemodialysis | 17 (13.2) | 8 (32) | .03 |
| CRRT | 8 (6.2) | 10 (40) | <.001 |
| Previous ESBL-E colonizationb | 40 (31.2) | 10 (40) | .48 |
| Severity of clinical presentation | |||
| No sepsis | 52 (40.6) | 0 | <.001 |
| Sepsis | 54 (42.2) | 5 (20) | .04 |
| Septic shock | 22 (17.2) | 20 (80) | <.001 |
| ICU admission due to ESBL-E infection | 62 (48.4) | 12 (48) | 1.0 |
| Type of infection | |||
| Nosocomial pneumoniae | 33 (25.7) | 13 (25) | 1.0 |
| ABSSSI | 25 (19.5) | 0 | .01 |
| cUTI | 31 (24.2) | 3 (12) | .29 |
| cIAI | 19 (14.8) | 6 (24) | .24 |
| Bone infection | 4 (3.1) | 1 (4) | 1.0 |
| Primary bacteremia | 14 (10.9) | 2 (8) | 1.0 |
| Other infectionsd | 2 (1.5) | 0 | 1.0 |
| Concomitant ESBL-E bacteremia | 35 (27.3) | 12 (48) | .05 |
| Life-threatening infection | 81 (63.2) | 10 (40) | .04 |
| Polymicrobial infection | 24 (18.7) | 7 (28) | .28 |
| Antibiotics before C/T treatment | |||
| Received antibiotics before C/T for current infection | 35 (27.3) | 17 (68) | <.001 |
| No. of antibiotics received, median (range) | 1 (1–3) | 2 (1–4) | .08 |
| Days of antibiotic therapy, median (range) | 6 (3–13) | 7 (4–15) | .07 |
| C/T treatment | |||
| Empiric treatment | 46 (35.9) | 0 | <.001 |
| Combination therapy | 23 (17.9) | 3 (12) | .57 |
| Time from infection onset to C/T administration, median (IQR), d | 4 (1–6) | 7 (5–14) | <.001 |
| Days of treatment, median (range) | 11 (6–22) | 17 (7–29) | <.001 |
| Extended infusion | 32 (25) | 2 (8) | .06 |
| Continuous infusion | 9 (7) | 2 (8) | 1.0 |
| Intermittent infusion | 87 (67.9) | 21 (84) | .14 |
| Standard dosage (or adjusted according to creatinine clearance)f | 95 (74.2) | 20 (80) | .62 |
| Off-label dosage | 33 (25.7) | 5 (20) | .68 |
| Adequate source control of infection | 43/57 (75.4) | 4/57 (7.1) | <.001 |
Abbreviations: ABSSSI, acute bacterial skin and skin-structure infection; C/T, ceftolozane/tazobactam; cIAI, complicated intra-abdominal infection; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; cUTI, complicated urinary tract infection; ESBL-E, extended-spectrum β-lactamase Enterobacterales; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation.
aData are No. (%) unless otherwise stated.
bWithin previous 30 days.
cAbsolute neutrophil count <500/mm3.
dOther infections include central venous catheter–related bacteremia (n = 1) and community-acquired pneumonia (n = 1).
eNosocomial pneumonia was divided into 26 patients with hospital-acquired pneumonia and 7 with ventilator-associated pneumonia among patients with clinical success; 6 patients with hospital-acquired pneumonia and 7 with ventilator-associated pneumonia among patients with clinical failure.
fAugmented renal clearance was reported in 4 patients with clinical success and 2 patients with clinical failure.
Table 4.
Description of Patients Who Experienced Clinical Failure With Ceftolozane/Tazobactam Therapy
| Case | Type of Infection | Concomitant BSI | Therapy Before C/T | Dose of C/T for the Present Infection | Concomitant Isolates | Additional Information | Reason for Clinical Failure |
|---|---|---|---|---|---|---|---|
| 1 | HAP | Yes | Cefepime | Off-label dosage | No | Septic shock, CRRT | Died |
| 2 | HAP | Yes | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | Acinetobacter baumannii | Septic shock | Died |
| 3 | HAP | Yes | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | Acinetobacter baumannii | Septic shock, CRRT | Lack of clinical response |
| 4 | HAP | Yes | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | No | Septic shock | Died |
| 5 | HAP | Yes | Piperacillin/tazobactam | Off-label dosage | No | Septic shock | Lack of clinical response |
| 6 | HAP | Yes | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | No | CRRT | Lack of clinical response |
| 7 | VAP | Yes | Piperacillin/tazobactam | Off-label dosage | No | Septic shock | Died |
| 8 | VAP | Yes | Piperacillin/tazobactam | Off-label dosage | No | Septic shock | Lack of clinical response |
| 9 | VAP | Yes | Meropenem | Off-label dosage | No | CRRT | Lack of clinical response |
| 10 | VAP | Yes | Meropenem | Standard dosage (or adjusted according to creatinine clearance) | No | Septic shock | Died |
| 11 | VAP | No | Meropenem | Standard dosage (or adjusted according to creatinine clearance) | Acinetobacter baumannii | Septic shock, CRRT | Died |
| 12 | VAP | No | Meropenem | Standard dosage (or adjusted according to creatinine clearance) | No | Septic shock | Died |
| 13 | VAP | No | Cefepime | Standard dosage (or adjusted according to creatinine clearance) | No | Septic shock | Died |
| 14 | cUTI | No | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock, CRRT | Died |
| 15 | cUTI | No | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock, CRRT | Died |
| 16 | cUTI | No | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock | Lack of clinical response |
| 17 | cIAI | Yes | Ceftriaxone+metronidazole | Augmented renal clearance | Acinetobacter baumannii | Inadequate source control of infection | Lack of clinical response |
| 18 | cIAI | Yes | Levofloxacin+metronidazole | Standard dosage (or adjusted according to creatinine clearance) | Enterococcus faecium vancomycin-resistant | Inadequate source control of infection, inadequate antimicrobial therapy | Lack of clinical response |
| 19 | cIAI | No | Piperacillin/tazobactam | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock | Lack of clinical response |
| 20 | cIAI | No | Ceftriaxone+metronidazole | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock, CRRT | Died |
| 21 | cIAI | No | Ceftriaxone+metronidazole | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, CRRT | Died |
| 22 | cIAI | No | Cefepime+metronidazole | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock, CRRT | Died |
| 23 | Bone infection | No | Cefepime+levofloxacin | Standard dosage (or adjusted according to creatinine clearance) | No | Inadequate source control of infection, septic shock | Lack of clinical response |
| 24 | Primary bacteremia | - | Cefepime | Standard dosage (or adjusted according to creatinine clearance) | MRSA | Septic shock | Died |
| 25 | Primary bacteremia | - | Ceftriaxone | Augmented renal clearance | MRSA | Septic shock | Died |
Abbreviations: CRRT, continuous renal replacement therapy; HAP, hospital-acquired pneumonia; IAI, intra-abdominal infection; MRSA, methicillin-resistant Staphylococcus aureus; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Finally, in multivariate analysis, Charlson comorbidity index >4 (odds ratio [OR], 2.3; 95% confidence interval [CI], 1.9–3.5; P = .02), septic shock (OR, 6.2; 95% CI, 3.8–7.9; P < .001), and continuous renal replacement therapy (OR, 3.1; 95% CI, 1.9–5.3; P = .001) were independently associated with clinical failure, whereas empiric therapy displaying in vitro activity (OR, 0.12; 95% CI, 0.01–0.34; P < .001) and adequate source control of infection (OR, 0.42; 95% CI, 0.14–0.55; P < .001) were associated with clinical success (Table 5).
Table 5.
Multivariate Analysis of Risk Factors for Clinical Failure of C/T Therapy Among Patients With Enterobacterales Infection
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Charlson comorbidity index >4 | 2.3 | 1.9–3.5 | .02 |
| Septic shock | 6.2 | 3.8–7.9 | <.001 |
| Empiric therapy displaying in vitro activity | 0.12 | 0.01-0-34 | <.001 |
| CRRT | 3.1 | 1.9–5.3 | .001 |
| Adequate source control of the infection | 0.42 | 0.14–0.55 | <.001 |
Abbreviations: C/T, ceftolozane/tazobactam; CI, confidence interval; CRRT, continuous renal replacement therapy; OR, odds ratio.
DISCUSSION
This study reports the largest clinical experience with C/T therapy for the treatment of serious ESBL-E infection published so far. We showed that C/T is an effective drug for treating different types of ESBL-E infections. This analysis also indicated that baseline conditions (expressed by a higher Charlson comorbidity index score), severity of clinical presentation at the time of infection (as demonstrated by the percentage of septic patients in the study population), and CRRT are associated with a significantly increased risk of clinical failure, whereas empiric therapy displaying in vitro activity and adequate source control of infection are associated with clinical success. Of importance, clinical success was observed in 83.7% of patients; over 65% of patients developed sepsis (38.6%) or septic shock (27.5%) at the time of infection.
As previously reported in the literature [1–4], severe infections caused by ESBL-E are associated with high rates of treatment failure and increased mortality, particularly when appropriate antimicrobial therapy is delayed. The role of carbapenems, considered the first choice for the treatment of these infections [20, 21], was redefined also for the high incidence of carbapenem-resistant Enterobacterales strains observed in the last few years. Attention is now focused on promotion of carbapenem-sparing strategies and evaluation of the efficacy of other drugs, like BLBLI combinations that remain active against a considerable proportion of ESBL-E; however, the role of these drugs is controversial for the treatment of serious infections due to ESBL pathogens [22, 23].
In a pooled post hoc analysis including patients from both ASPECT-cIAI [8] and ASPECT-cUTI [7] who had an ESBL-E in their baseline cultures (150/1346, 11.1%), clinical cure rates were 97.4% for C/T (76/78), 82.6% for levofloxacin, and 88.5% for meropenem (23/26) [24]. Interestingly, in our population a high clinical success rate was also retained in patients who received C/T as salvage therapy (76.2%), as well as those with life-threatening infection (63.2%). Our data are in line with those of the recently published paper ASPECT-NP [14], studying patients with severe intubated pneumonia, which demonstrated a microbiological success rate in infections caused by ESBL-E of 57% for C/T compared with 61.6% for meropenem in a setting of ESBL-E resistant to C/T, for a rate of 38%. This study caused EUCAST to increase the breakpoints of C/T from 1 mg/L to 2 mg/L [25].
These clinical data, in line with the high in vitro activity against ESBL producers reported in the literature [5], configure for C/T a possible role (also in empiric therapy) as a carbapenem-sparing agent, especially in a geographical area with high spread of gram-negative carbapenem-resistant strains [6] and in patients with a lower risk of infection caused by a carbapenem-resistant strain [26]. Of importance, no clinical failure was reported among patients with C/T administered in empiric therapy when compared with patients for whom C/T was used in targeted or rescue therapy (P < .001). This observation is in line with our data from multivariate analysis, which showed that as empiric therapy, C/T displayed in vitro activity associated with clinical success in this study population. Of interest, according to etiology, a successful clinical outcome was reported in a high percentage (90.5%) of cases with Escherichia coli infections, while a lower rate of clinical success was observed for those with Klebsiella pneumoniae (84.4%) and Enterobacter spp. infections (68.2%), in line with previous observations [24].
On this basis, reflecting an unmet need for licensed treatment options, C/T is frequently used for off-label indications, and data regarding how C/T performs overall in the treatment of severe infections are expanding [11]. Recently, a randomized, controlled, double-blind, phase 3 noninferiority trial (ASPECT-NP) compared C/T (3 g every 8 hours) with meropenem for treatment of nosocomial pneumonia. Of importance, high-dose C/T was efficacious and well tolerated for gram-negative nosocomial pneumonia in this critically ill population, comprising mechanically ventilated patients [14].
The current study confirms a previous observation on an association between clinical failure and receipt of CRRT during C/T therapy [12]. Optimal dosing of C/T in patients receiving CRRT is an unresolved issue, and no dosing recommendations are currently available in this specific setting [6]. Previous preliminary reports [16,17] have suggested that a standard dosage of 1.5 g q8h should ensure appropriate C/T exposure for the treatment of pneumonia in patients undergoing CRRT. However, considering that all patients with CRRT in the current study received a C/T dosage of 1.5 g q8h, we suggest consideration of an increased posology of C/T in these patients or, if possible, routine performance of therapeutic drug monitoring.
Finally, no differences in clinical response rates between patients treated with combination therapy and those treated with monotherapy were observed in this study (P = .34). However, the low number of patients receiving combination therapy (n = 26) prevents us from drawing further conclusions with regard to the benefit of combination treatment, especially in patients with high risk of mortality such as those presenting with septic shock. Moreover, another interesting finding was the low number of observed AEs during treatment. Only 2 patients developed mild AEs, although threating physicians interrupted treatment in both patients. In pivotal clinical trials, gastrointestinal side effects and abnormal liver enzyme increase were also the most frequent AEs described [7, 8].
This study has several limitations that should be addressed. First, it was an observational, retrospective study; therefore, we may not have been able to control for all measured and unmeasured variables that may have had a clinical impact on patient evolution. Nevertheless, our series of ESBL-E infections treated with C/T is the largest real-life experience ever reported in literature. Second, C/T was mainly administered as second- or third-line therapy, and the role of prior therapy on clinical outcome is unclear. Third, susceptibility testing was performed at each individual center, and we do not have molecular analysis to determine the presence of enzymes associated with antibiotic resistance in the isolates. Fourth, we did not perform antibiotic blood levels, and we cannot exclude that clinical failure in some critically ill patients could be related to the higher clearance of β-lactams [27–29], considering also lack of information about other variables that may influence the outcome, such as circulatory management. Finally, no data were recorded on long-term survival over 30 days; thus, we cannot provide more consistent information on the risk of recurrence of infection and emergence of strains resistant to C/T therapy.
In conclusion, our data showed that C/T could be a valid option in empiric and/or targeted therapy in patients with severe infections caused by ESBL-producing Enterobacterales. The decision about C/T in empiric therapy was based on clinical judgment at participating centers. In our opinion, the reason for the use of C/T was based on knowledge of high spread of ESBL strains in the geographical area involved in this study, the high rate of hospital-acquired infection, and the severity of the clinical condition (as expressed by Charlson comorbidity index score and/or rate of septic shock). Clinicians should be aware of the risk of clinical failure with C/T therapy in septic patients receiving CRRT. The results of this study are relevant to physicians who attend patients with a wide variety of diseases and severity of illness.
Acknowledgments
The authors acknowledge as co-authors all of the following CEFTABUSE Study Group members: Matteo Bassetti, Antonio Vena, Daniele Roberto Giacobbe, Claudio Viscoli (Department of Health Sciences, University of Genoa, Genoa, Italy); Alessandro Russo, Marco Falcone, Giusy Tiseo, Francesco Menichetti, Stefano Verdenelli, Silvia Fabiani (Infectious Diseases Clinic, Nuovo Santa Chiara University Hospital, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy); Nadia Castaldo, Davide Pecori, Alessia Carnellutti, Filippo Givone, Elena Graziano, Maria Merelli, Barbara Cadeo, Maddalena Peghin (Infectious Diseases Division, Santa Maria della Misericordia University Hospital, Udine, Italy); Maddalena Giannella, Renato Pascale, Pierlugi Viale (Infectious Diseases Unit, Department of Medical and Surgical Sciences, Policlinico Sant’Orsola Malpighi, University of Bologna, Bologna, Italy); Annamaria Cattelan, Ludovica Cipriani, Davide Coletto (Unit of Infectious Diseases, Department of Internal Medicine, Azienda Ospedaliera-Universitaria di Padova, Padua, Italy); Cristina Mussini, Margherita Digaetano (Division of Infectious Diseases, University of Modena, Modena, Italy); Carlo Tascini, Novella Carannante (First Division of Infectious Diseases, Cotugno Hospital, AORN dei Colli, Naples, Italy); Claudio Maria Mastroianni, Russo Gianluca, Alessandra Oliva, Maria Rosa Ciardi, Camilla Ajassa, Tiziana Tieghi (Infectious Diseases Unit, Sapienza University of Rome, Latina, Italy, Department of Public Health and Infectious Diseases, Policlinico Umberto I, Sapienza University of Rome, Rome, Italy; Polo Pontino, Ospedale Santa Maria Goretti, ‘Sapienza’ University of Rome, Latina, Italy); Mario Tumbarello, Angela Raffaella Losito, Francesca Raffaelli (UOC Malattie Infettive, Fondazione Policlinico Universitario A. Gemelli IRCCS - Istituto Malattie Infettive, Università Cattolica del Sacro Cuore, Rome, Italy); Paolo Grossi, Cristina Rovelli (Department of Infectious and Tropical Diseases, University of Insubria, Ospedale di Circolo-Fondazioni Macchi, Varese, Italy); Stefania Artioli, Giorgia Caruana (Infectious Diseases and Hepatology Unit, Sant’Andrea Hospital La Spezia, La Spezia, Italy); Roberto Luzzati, Giulia Bontempo (Infectious Diseases Division, University Hospital of Trieste, Trieste, Italy); Nicola Petrosillo, Alessandro Capone (Clinical and Research Department for Infectious Diseases, Unit of Systemic and Immunedepression-Associated Infections, National Institute for Infectious Diseases L. Spallanzani, Rome, Italy); Giuliano Rizzardini, Massimo Coen, Matteo Passerini (Department of Infectious Diseases I, L. Sacco University Hospital, Milan, Italy); Antonio Mastroianni and Giuliana Guadagnino (Infectious Diseases Unit, ‘Annunziata’ Hospital, Cosenza, Italy); Filippo Urso (Hospital Pharmacy, “Annunziata” Hospital, Cosenza, Italy); Guglielmo Borgia, Ivan Gentile, Alberto Enrico Maraolo (Department of Clinical Medicine and Surgery, Section of Infectious Diseases, University of Naples Federico II, Naples, Italy); Massimo Crapis, Sergio Venturini (Azienda per l’Assistenza Sanitaria n 5 Friuli Occidentale Ringgold Standard Institution – Infectious Diseases Unit, Pordenone, Friuli-Venezia Giulia, Italy); Giustino Parruti, Francesca Trave (Infectious Diseases Unit, Pescara General Hospital, Pescara, Italy); Gioacchino Angarano, Sergio Carbonara, Michele Fabiano Mariani (Clinic of Infectious Diseases, University of Bari, Bari, Italy); Massimo Girardis (Department of Anesthesia and Intensive Care, University Hospital of Modena, Modena, Italy); Antonio Cascio, Claudia Gioe (Infectious Diseases Division, Department of Health Promotion Sciences and Mother and Child Care ‘G. D’Alessandro,’ University of Palermo, Palermo, Italy); Marco Anselmo, Emanuele Malfatto (Infectious Diseases Unit, S. Paolo Hospital, ASL 2 Savona, Italy).
Ethical approval. The Internal Review board of Medical Area (DAME) of Azienda Ospedaliera Universitaria Integrata di Udine (Udine, Italy) approved this study (18/I.R.B_Bassetti_18), which waived the requirement of informed consent owing to the retrospective design of the study.
Financial support. This work was supported by a research grant awarded under the Investigator Initiated Study Program of Merck & Co.
Potential conflicts of interest. M.B. serves on scientific advisory boards for Angelini, AstraZeneca, Bayer, Cubist, Pfizer, Menarini, MSD, Nabriva, Paratek, Roche, Shionogi, Tetraphase, The Medicine Company, and Astellas Pharma Inc. and has received funding for travel or speaker honoraria from Algorithm, Angelini, Astellas Pharma Inc., AstraZeneca, Cubist, Pfizer, MSD, Gilead Sciences, Menarini, Novartis, Ranbaxy, and Teva; CT has received funds for speaking at symposia organized on behalf of Pfizer, Gilead, Novartis, Merck, Angelini, Thermo Fisher, Biotest, and Astellas. All other authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the CEFTABUSE Study Group:
Matteo Bassetti, Antonio Vena, Daniele Roberto Giacobbe, Claudio Viscoli, Alessandro Russo, Marco Falcone, Giusy Tiseo, Francesco Menichetti, Stefano Verdenelli, Silvia Fabiani, Nadia Castaldo, Davide Pecori, Alessia Carnellutti, Filippo Givone, Elena Graziano, Maria Merelli, Barbara Cadeo, Maddalena Peghin, Maddalena Giannella, Renato Pascale, Pierlugi Viale, Annamaria Cattelan, Ludovica Cipriani, Davide Coletto, Cristina Mussini, Margherita Digaetano, Carlo Tascini, Novella Carannante, Claudio Maria Mastroianni, Russo Gianluca, Alessandra Oliva, Maria Rosa Ciardi, Camilla Ajassa, Tiziana Tieghi, Mario Tumbarello, Angela Raffaella Losito, Francesca Raffaelli, Paolo Grossi, Cristina Rovelli, Stefania Artioli, Giorgia Caruana, Roberto Luzzati, Giulia Bontempo, Nicola Petrosillo, Alessandro Capone, Giuliano Rizzardini, Massimo Coen, Matteo Passerini, Antonio Mastroianni, Giuliana Guadagnino, Filippo Urso, Guglielmo Borgia, Ivan Gentile, Alberto Enrico Maraolo, Massimo Crapis, Sergio Venturini, Giustino Parruti, Francesca Trave, Gioacchino Angarano, Sergio Carbonara, Michele Fabiano Mariani, Massimo Girardis, Antonio Cascio, Claudia Gioe, Marco Anselmo, and Emanuele Malfatto
References
- 1. Rodríguez-Baño J, Navarro MD, Romero L, et al. . Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis 2006; 43:1407–14. [DOI] [PubMed] [Google Scholar]
- 2. Kang CI, Kim SH, Park WB, et al. . Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 2005; 49:760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tumbarello M, Sanguinetti M, Montuori E, et al. . Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007; 51:1987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russo A, Falcone M, Gutiérrez-Gutiérrez B, et al. ; REIPI/ESGBIS/INCREMENT investigators Predictors of outcome in patients with severe sepsis or septic shock due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Int J Antimicrob Agents 2018; 52:577–85. [DOI] [PubMed] [Google Scholar]
- 5. Pfaller MA, Bassetti M, Duncan LR, Castanheira M. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: report from an antimicrobial surveillance programme (2012-15). J Antimicrob Chemother 2017; 72:1386–95. [DOI] [PubMed] [Google Scholar]
- 6. Giacobbe DR, Bassetti M, De Rosa FG, et al. ; ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Ceftolozane/tazobactam: place in therapy. Expert Rev Anti Infect Ther 2018; 16:307–20. [DOI] [PubMed] [Google Scholar]
- 7. Wagenlehner FM, Umeh O, Steenbergen J, et al. . Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 2015; 385:1949–56. [DOI] [PubMed] [Google Scholar]
- 8. Solomkin J, Hershberger E, Miller B, et al. . Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 2015; 60:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haidar G, Philips NJ, Shields RK, et al. . Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munita JM, Aitken SL, Miller WR, et al. . Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis 2017; 65:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bassetti M, Castaldo N, Cattelan A, et al. ; CEFTABUSE Study Group Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: a multicentre nationwide clinical experience. Int J Antimicrob Agents 2019; 53:408–15. [DOI] [PubMed] [Google Scholar]
- 12. European Centre for Disease Prevention and Control. Annual Epidemiological Report on Communicable Diseases in Europe. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2014. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/AER-VPD-IBD-2014.pdf. Accessed 30 November 2019. [Google Scholar]
- 13. Singer M, Deutschman CS, Seymour CW, et al. . The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kollef MH, Nováček M, Kivistik Ü, et al. . Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2019; 19:1299–311. [DOI] [PubMed] [Google Scholar]
- 15. Bremmer DN, Nicolau DP, Burcham P, et al. . Ceftolozane/tazobactam pharmacokinetics in a critically ill adult receiving continuous renal replacement therapy. Pharmacotherapy 2016; 36:e30–3. [DOI] [PubMed] [Google Scholar]
- 16. Kuti JL, Ghazi IM, Quintiliani R Jr, et al. . Treatment of multidrug-resistant Pseudomonas aeruginosa with ceftolozane/tazobactam in a critically ill patient receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 2016; 48:342–3. [DOI] [PubMed] [Google Scholar]
- 17. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356:1255–9. [DOI] [PubMed] [Google Scholar]
- 18. Stürenburg E, Sobottka I, Noor D, et al. . Evaluation of a new cefepime-clavulanate ESBL Etest to detect extended-spectrum beta-lactamases in an Enterobacteriaceae strain collection. J Antimicrob Chemother 2004; 54:134–8. [DOI] [PubMed] [Google Scholar]
- 19. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available at: http://www.eucast.org/clinical_breakpoints/. Accessed 7 January 2020.
- 20. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005; 18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 2008; 8:159–66. [DOI] [PubMed] [Google Scholar]
- 22. Tamma PD, Han JH, Rock C, et al. ; Antibacterial Resistance Leadership Group Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 2015; 60:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PNA, Tambyah PA, Lye DC, et al. ; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Popejoy MW, Paterson DL, Cloutier D, et al. . Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: a pooled analysis of phase 3 clinical trials. J Antimicrob Chemother 2017; 72:268–72. [DOI] [PubMed] [Google Scholar]
- 25. European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/2019/EUCAST_General_Consultation_on_ceftolozane-tazobactam_breakpoints_20191101.pdf. Accessed 28 December 2019.
- 26. Giannella M, Trecarichi EM, De Rosa FG, et al. . Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect 2014; 20:1357–62. [DOI] [PubMed] [Google Scholar]
- 27. Carrié C, Petit L, d’Houdain N, et al. . Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents 2018; 51:443–9. [DOI] [PubMed] [Google Scholar]
- 28. Udy AA, Dulhunty JM, Roberts JA, et al. ; BLING-II Investigators; ANZICS Clinical Trials Group Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents 2017; 49:624–30. [DOI] [PubMed] [Google Scholar]
- 29. Tsai D, Stewart P, Goud R, et al. . Total and unbound ceftriaxone pharmacokinetics in critically ill Australian indigenous patients with severe sepsis. Int J Antimicrob Agents 2016; 48:748–52. [DOI] [PubMed] [Google Scholar]