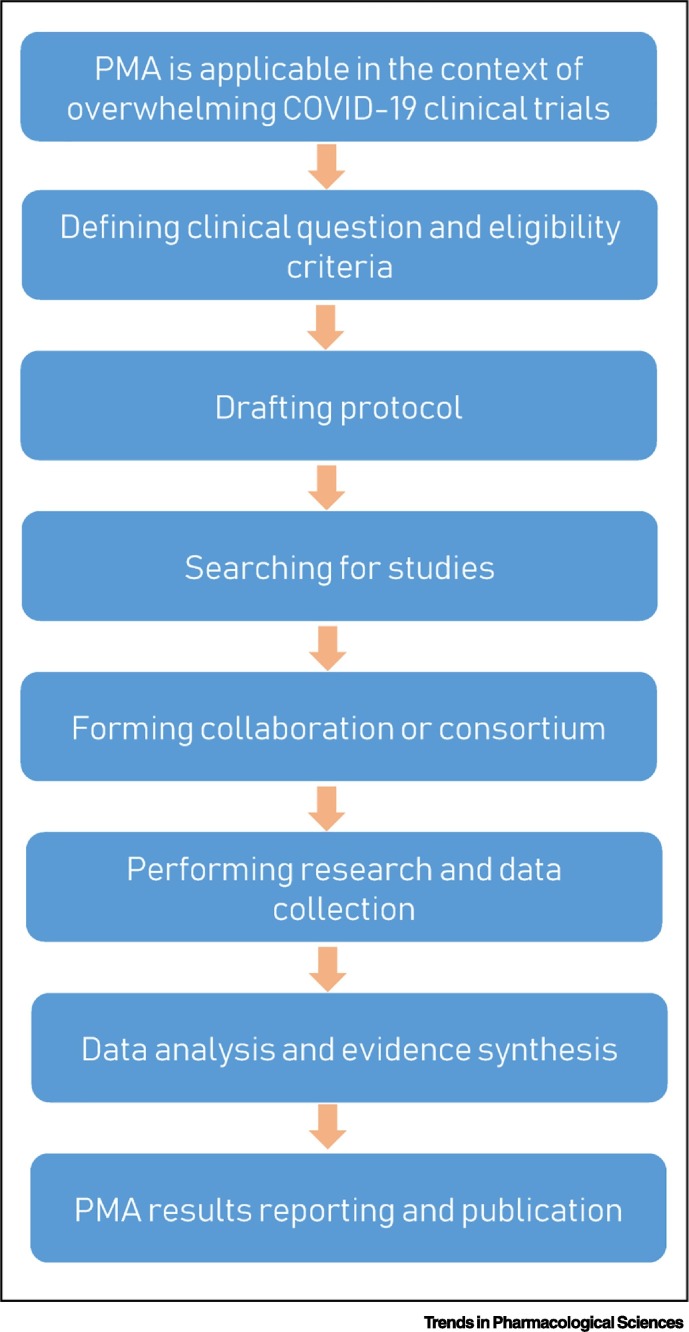
Figure 1.
A Workflow for Conducting Prospective Meta-Analyses (PMAs) in the Context of Overwhelming Coronavirus 2019 (COVID-19) Clinical Trials.
Prospectively forming a collaboration or consortium of PMA to pool multiple eligible studies that aim to address the same clinical question will likely generate reliable data for guiding clinical management and regulatory decision-making. This will not affect the individual studies and will not prevent the publication of the results of those individual studies.