Key Points
Question
Is maternal antenatal corticosteroid treatment associated with mental and behavioral disorders in children?
Findings
In this population-based retrospective cohort study that included 670 097 children, exposure to maternal antenatal corticosteroid treatment, compared with nonexposure, was significantly associated with mental and behavioral disorders in children (hazard ratio, 1.33).
Meaning
These findings may help inform decisions about maternal antenatal corticosteroid treatment.
Abstract
Importance
Maternal antenatal corticosteroid treatment is standard care to accelerate fetal maturation when birth before 34 weeks is imminent. Recently, expansion of the indications beyond 34 gestational weeks has been debated. However, data about long-term outcomes remain limited, especially among infants who after treatment exposure are born at term.
Objective
To study if antenatal corticosteroid treatment is associated with mental and behavioral disorders in children born at term (≥37 weeks 0 days’ gestation) and preterm (<37 weeks 0 days’ gestation) and if unmeasured familial confounding explains these associations.
Design, Setting, and Participants
Population-based retrospective cohort study using nationwide registries of all singleton live births in Finland surviving until 1 year and a within-sibpair comparison among term siblings. Children were born between January 1, 2006, and December 31, 2017, and followed up until December 31, 2017.
Exposures
Maternal antenatal corticosteroid treatment.
Main Outcomes and Measures
Primary outcome was any childhood mental and behavioral disorder diagnosed in public specialized medical care settings.
Results
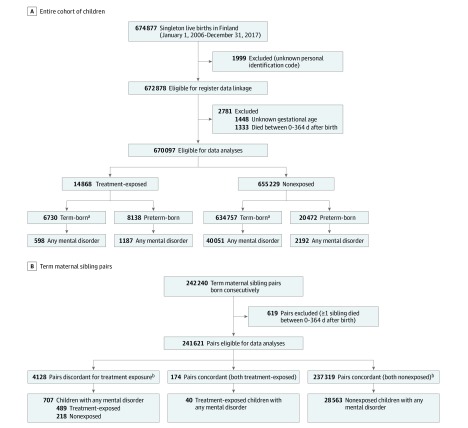
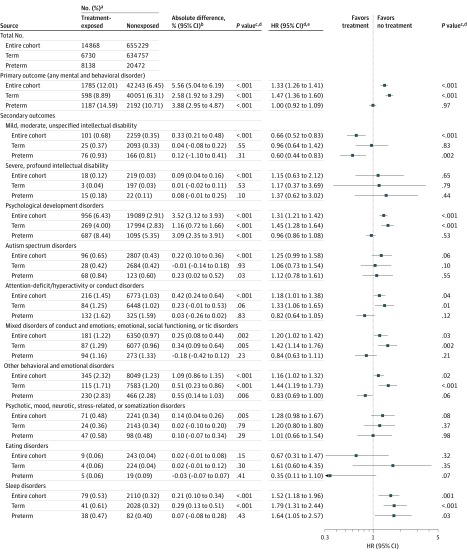
Of the 674 877 singleton children born in Finland during the study period, 670 097 were eligible for analysis. The median length of follow-up was 5.8 (interquartile-range, 3.1-8.7) years. Of the 14 868 (2.22%; 46.1% female) corticosteroid treatment–exposed children, 6730 (45.27%) were born at term and 8138 (54.74%) were born preterm; of the 655 229 (97.78%; 48.9% female) nonexposed children, 634 757 (96.88%) were born at term and 20 472 (3.12%) were born preterm. Among the 241 621 eligible term-born maternal sibpairs nested within this population, 4128 (1.71%) pairs were discordant for treatment exposure. Treatment exposure, compared with nonexposure, was significantly associated with higher risk of any mental and behavioral disorder in the entire cohort of children (12.01% vs 6.45%; absolute difference, 5.56% [95% CI, 5.04%-6.19%]; adjusted hazard ratio [HR], 1.33 [95% CI, 1.26-1.41]), in term-born children (8.89% vs 6.31%; absolute difference, 2.58% [95% CI, 1.92%-3.29%]; HR, 1.47 [95% CI, 1.36-1.69]), and when sibpairs discordant for treatment exposure were compared with sibpairs concordant for nonexposure (6.56% vs 4.17% for within-sibpair differences; absolute difference, 2.40% [95% CI, 1.67%-3.21%]; HR, 1.38 [95% CI, 1.21-1.58]). In preterm-born children, the cumulative incidence rate of any mental and behavioral disorder was also significantly higher for the treatment-exposed compared with the nonexposed children, but the HR was not significant (14.59% vs 10.71%; absolute difference, 3.38% [95% CI, 2.95%-4.87%]; HR, 1.00 [95% CI, 0.92-1.09]).
Conclusions and Relevance
In this population-based cohort study, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children. These findings may help inform decisions about maternal antenatal corticosteroid treatment.
This population cohort study uses national Finnish birth registry data to assess associations between antenatal corticosteroid treatment to accelerate fetal maturation and mental and behavioral disorders in term and preterm children.
Introduction
Maternal antenatal corticosteroid treatment to accelerate fetal maturation is standard care before 34 weeks 0 days’ gestation when there is a risk of delivery within 7 days.1,2,3 In infants born before 34 weeks 0 days, this treatment reduces risks of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, need for mechanical ventilation, systemic infections, and death.1,2,3,4 Because treatment also reduces neonatal morbidity when administered beyond 34 weeks,5,6,7,8 updates to US guidelines in 2016 recommended treatment for pregnant women between 34weeks 0 days to 36 weeks 6 days who are at risk for preterm delivery within 7 days and who have not received a previous course of antenatal corticosteroids.1 This recommendation may lead to an increase in the number of treatment-exposed children: in the US, 2.76% of all infants are born before 34 weeks 0 days, while 7.17% are born between 34 weeks 0 days and 36 weeks 6 days.9
While the short-term benefits for infant morbidity and mortality are known, the long-term outcomes of treatment exposure remain uncertain. The effects on child neurodevelopment are of particular concern, as corticosteroids cross the placenta and the blood-brain barrier and may harm fetal brain development.10,11,12,13 Moreover, as the prediction of timing of birth is difficult, a large number of treatment-exposed children are not delivered within 7 days and go on to be born at term (≥37 weeks 0 days).5,14 However, data on neurodevelopmental outcomes of treatment-exposed term-born children remain limited. In addition, to our knowledge, no population-based studies testing associations between treatment exposure and child neurodevelopment or studies testing if unmeasured familial confounding would explain any associations have been performed.
To address these knowledge gaps, this population-based study examined whether treatment exposure was associated with a risk of childhood mental and behavioral disorders, and whether this risk was similar in infants born at term and preterm.
Methods
Study Population
Unique personal identification numbers assigned to all Finnish citizens and permanent residents were used to merge information from different registers kept at the Finnish Institute for Health and Welfare, the statutory statistical authority for social and health care data in Finland, with permission from the registries. The data analysis was conducted as an internal procedure of the Finnish Institute for Health and Welfare, and registered individuals were not contacted. According to Finnish legislation, neither institutional review board approval nor informed consent is required in such studies.
All singleton pregnancies ending in a live birth in Finland between January 1, 2006, and December 31, 2017, were identified from the Medical Birth Register.15 This register includes information since 1987 on all live births and stillbirths in Finland with gestational age of 22 weeks or more or birth weight 500 g or greater. Infants who had valid maternal and child personal identification codes for register data linkage, had data on gestational age, and survived until the end of the first year of life were eligible for data analyses. From this population, we also identified all consecutive maternal sibpairs born at term, including sibpairs discordant for maternal antenatal corticosteroid treatment exposure and concordant for treatment exposure and for nonexposure.
Maternal Antenatal Corticosteroid Treatment
Maternal antenatal corticosteroid treatment during pregnancy was recorded in the Medical Birth Register as treatment received or not. This register does not include information on the number or timing of treatments. The treatment recommended by Finnish national guidelines consisted of betamethasone (12 mg) administered twice, 24 hours apart throughout the study period. Until 2009, treatment was recommended up to 34 weeks 0 days (32 weeks 0 days in case of premature rupture of membranes); after 2009, treatment was recommended up to 34 weeks 6 days and, in select cases, later (eg, fetal hydrops; maternal disorder warranting cesarean delivery). Repeated treatments were not recommended before 2009; after 2009, 1 repeat course could be considered when the risk of respiratory distress was high.16
The treatment recorded in the Medical Birth Register showed high agreement with treatment recorded in patient case reports in 2 samples nested within our study population: in 1041 and 771 women giving birth to a singleton child in 2006-2010 and 2012-2017 in Finland, the crude agreements were 97.3% and 98.8%, respectively (eTable 1 in the Supplement).
Childhood Mental and Behavioral Disorders
Disorder diagnoses, as primary or secondary diagnoses, came from the Finnish Care Register for Health Care using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes on all hospital inpatient (since 1969) and outpatient (since 1998) treatments by physicians in specialized medical care. Validation studies have indicated that the Finnish Care Register for Health Care has high validity for psychiatric diagnoses.17,18
Any childhood mental and behavioral disorder was studied as the primary outcome. Secondary outcomes included 10 specific mental and behavioral disorders grouped as described,19 based on symptomatic similarities (eTable 2 in the Supplement).
Covariates
Based on previous literature, we identified covariates that were associated with either antenatal corticosteroids, preterm birth, and/or childhood mental and behavioral disorders.1,2,3,4,14,20,21 Of these, the Medical Birth Register provided data on child birth year, sex, 1-minute and 5-minute Apgar score, admission to a neonatal intensive care unit, weight and gestational age at birth, and maternal age at delivery, parity, mode of delivery, smoking during pregnancy, prepregnancy body mass index (calculated from weight and height verified by measurement in the first antenatal clinic visit between 7-10 gestational weeks), premature rupture of membranes (ICD-10 code O42), gestational diabetes (ICD-10 code O24), and hypertension in pregnancy (ICD-10 codes O10, O13-O15). Data on maternal any lifetime mental disorder diagnosis, including disorders due to psychoactive substance use, came from the Finnish Care Register for Health Care (International Classification of Diseases, Ninth Revision codes 290-319 in 1987-1995 and ICD-10 codes F00–F99 in 1996-2017). Race or ethnicity are not recorded in Finnish registers, including the Medical Birth Register, because of European Union legislation (General Data Protection Regulation).
Statistical Analysis
Cox proportional hazards modeling estimated the associations between maternal antenatal corticosteroid treatment exposure and mental and behavioral disorders in the entire cohort, and in term-born and preterm-born children. Kaplan-Meier curves estimated the length of time from birth to the primary outcome, the first diagnosis of any mental and behavioral disorder, and the cumulative probability to remain diagnosis-free at the end of the follow-up. Length of time from birth to the primary outcome was also quantified as the median age (interquartile range) at the first diagnosis. These analyses were repeated for the 10 secondary outcomes. Because of the potential for type I error due to multiple comparisons, findings for the secondary outcomes should be interpreted as exploratory.
We performed sensitivity analyses on the primary outcome by excluding postterm births (≥42 weeks 0 days) from the analyses, as they may increase the risk for mental and behavioral disorders.22
In all within-sibpair analyses of term-born maternal siblings, within-sibpair differences of treatment exposure–discordant sibpairs were compared with within-sibpair differences of nonexposure-concordant sibpairs for the primary outcome.23 The first within-sibpair comparison included all maternal treatment exposure–discordant and nonexposure-concordant sibpairs in consecutive order. To account for reverse temporality, we then compared within-sibpair differences of treatment exposure–discordant sibpairs of which the younger was treatment exposed and the older was nonexposed with within-sibpair differences of nonexposure-concordant sibpairs of which both the younger and the older were nonexposed. To account for the dependence of sibling observations in our analyses, we compared within-sibpair differences of the first treatment exposure–discordant sibpairs with those of the first nonexposure-concordant sibpairs for each mother.
We present the associations in the entire cohort and term-born and preterm-born children as unadjusted and adjusted for all covariates. For within-sibpair comparisons, maternal age, parity, interpregnancy interval, any lifetime mental disorder diagnosis, child gestational age, sex, and any mental or behavioral disorder of the sibling who was not exposed to treatment were used as covariates.
Absolute risk differences of cumulative incidence rates and hazard ratios (HRs) with 95% CIs indicated effect sizes, and P < .05 (2-sided) was regarded as statistically significant. Proportional hazards assumptions were assessed on the plots of log(time) vs log (−log[survival]) and Schoenfeld tests and were verified as acceptable. Deviance and Martingale residuals were plotted, and these did not detect any outliers or nonlinearity. We conducted complete case analyses, as missing data in our study population were minimal. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Of 674 877 singleton infants born in Finland in the study period, 670 097 were eligible for analysis (Figure 1). Descriptive characteristics of the eligible study population of treatment-exposed and nonexposed children, followed up for a median of 5.8 (interquartile range, 3.1-8.7) years, are reported in the Table. Data on these characteristics were missing in 0.1% to 2.0% of the study population (Table). Of the 14 868 (2.22%; 46.1% female) treatment-exposed children, 6730 (45.27%) were born at term and 8138 (54.74%) born preterm; of the 655 229 (97.78%; 48.9% female) nonexposed children, 634 757 (96.88%) were born at term and 20 472 (3.12%) born preterm. Descriptive characteristics of term-born and preterm-born treatment-exposed and nonexposed children are reported in eTable 3 in the Supplement.
Figure 1. Participant Flow of the Population-Based Sample.
A, Entire cohort of children, including term (≥37 weeks 0 days’ gestation) and preterm-born (<37 weeks 0 days’ gestation) children. B, Cohort of term-born consecutive maternal sibling pairs.
aTerm group included children born postterm at 42 weeks 0 days or more.
bIn within-sibpair comparisons all maternal treatment exposure–discordant and nonexposure-concordant siblings are analyzed in consecutive order.
Table. Characteristics of the Study Population According to Maternal Antenatal Corticosteroid Treatment Exposure.
| No. (%)a | |||
|---|---|---|---|
| Entire cohort (N = 670 097) | Treatment-exposed (n = 14 868) | Nonexposed (n = 655 229) | |
| Children | |||
| Sex | |||
| Boys | 342 562 (51.1) | 8010 (53.9) | 334 552 (51.1) |
| Girls | 327 535 (48.9) | 6858 (46.1) | 320 677 (48.9) |
| Timing of birth | |||
| Preterm (<37 wk 0 d) | 28 610 (4.3) | 8138 (54.7) | 20472 (3.1) |
| Term (≥37 wk 0 d) | 641 487 (95.7) | 6730 (45.3) | 634 757 (96.9) |
| Birth weight, mean (SD), g | 3529 (527) | 2659 (950) | 3548 (496) |
| Apgar score (maximum at 1 and 5 min)b | |||
| 0-3 | 1364 (0.2) | 192 (1.3) | 1172 (0.2) |
| 4-6 | 9551 (1.4) | 1056 (7.1) | 8495 (1.3) |
| 7-10 | 658 226 (98.2) | 13 504 (90.8) | 644 722 (98.4) |
| Unknown | 956 (0.1) | 116 (0.8) | 840 (0.1) |
| Admission to NICU | |||
| No | 601 723 (89.8) | 7360 (49.5) | 594 363 (90.7) |
| Yes | 60 866 (10.2) | 7508 (50.5) | 60 866 (9.3) |
| Mothers | |||
| Age at delivery, mean (SD), y | 30.4 (5.3) | 30.6 (5.8) | 30.3 (5.3) |
| Parity | |||
| 0 | 278 639 (41.6) | 6750 (45.4) | 271 889 (41.5) |
| 1 | 227 064 (33.9) | 4458 (30.0) | 222 606 (34.0) |
| 2 | 98 070 (14.6) | 2081 (14.0) | 95 989 (14.6) |
| 3 | 33 874 (5.1) | 796 (5.4) | 33 078 (5.0) |
| ≥4 | 32 344 (4.8) | 783 (5.3) | 31 561 (4.8) |
| Unknown | 106 (0.02) | 0 | 106 (0.02) |
| Delivery mode | |||
| Vaginal | 563 805 (84.1) | 9324 (62.7) | 554 481 (84.6) |
| Cesarean | 106 292 (15.9) | 5544 (37.3) | 100 748 (15.4) |
| Prepregnancy BMI, mean (SD)c | 24.4 (4.9) | 24.5 (5.4) | 24.4 (4.9) |
| Unknown | 13 237 (2.0) | 161 (1.1) | 13 076 (2.0) |
| Premature rupture of membranesd | |||
| No | 650 286 (97.4) | 12 689 (83.3) | 640 200 (97.3) |
| Yes | 19 811 (3.0) | 2538 (16.7) | 17 451 (2.7) |
| Gestational diabetesd | |||
| No | 593 877 (88.6) | 1234 (83.2) | 581 503 (88.7) |
| Yes | 76 220 (11.4) | 2494 (16.8) | 73 726 (11.3) |
| Hypertensiond | |||
| No | 642 365 (95.9) | 13 335 (89.7) | 629 030 (96.0) |
| Yes | 27 732 (4.1) | 1533 (10.3) | 26 199 (4.0) |
| Any lifetime mental disorderd | |||
| No | 545 435 (81.6) | 10 895 (73.3) | 534 540 (81.6) |
| Yes | 124 662 (18.4) | 3973 (26.7) | 120 689 (18.4) |
| Smoking during pregnancy | |||
| No | 571 447 (85.3) | 12 101 (81.4) | 559 346 (85.4) |
| Yes | 98 650 (14.7) | 2767 (18.6) | 95 883 (14.6) |
Abbreviations: BMI, body mass index; NICU, neonatal intensive care unit.
Percentages may not total 100% because of rounding.
The Apgar score is calculated at 1 and 5 minutes after birth, uses skin color, pulse rate, reflexes, muscle tone, and respiratory effort to determine medical attention. Scores from 0 to 3 suggest a need for resuscitation, while scores of 7 or more are considered normal.
Calculated as weight in kilograms divided by height in meters squared.
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes: premature rupture of membranes, O42; gestational diabetes, O24; hypertension, O10, O13-O15; any lifetime mental disorder, including organic mental disorders, disorders due to psychoactive substance use, schizophrenia, schizotypal and delusional disorders, mood disorders, neurotic, stress-related and somatoform disorders, behavioral syndromes associated with physiological disturbances and physical factors, disorders of adult personality and behavior, intellectual disabilities, disorders of psychological development, behavioral and emotional disorders with onset usually occurring in childhood and adolescence, F00-F99.
Mental and Behavioral Disorders in Treatment-Exposed and Nonexposed Children
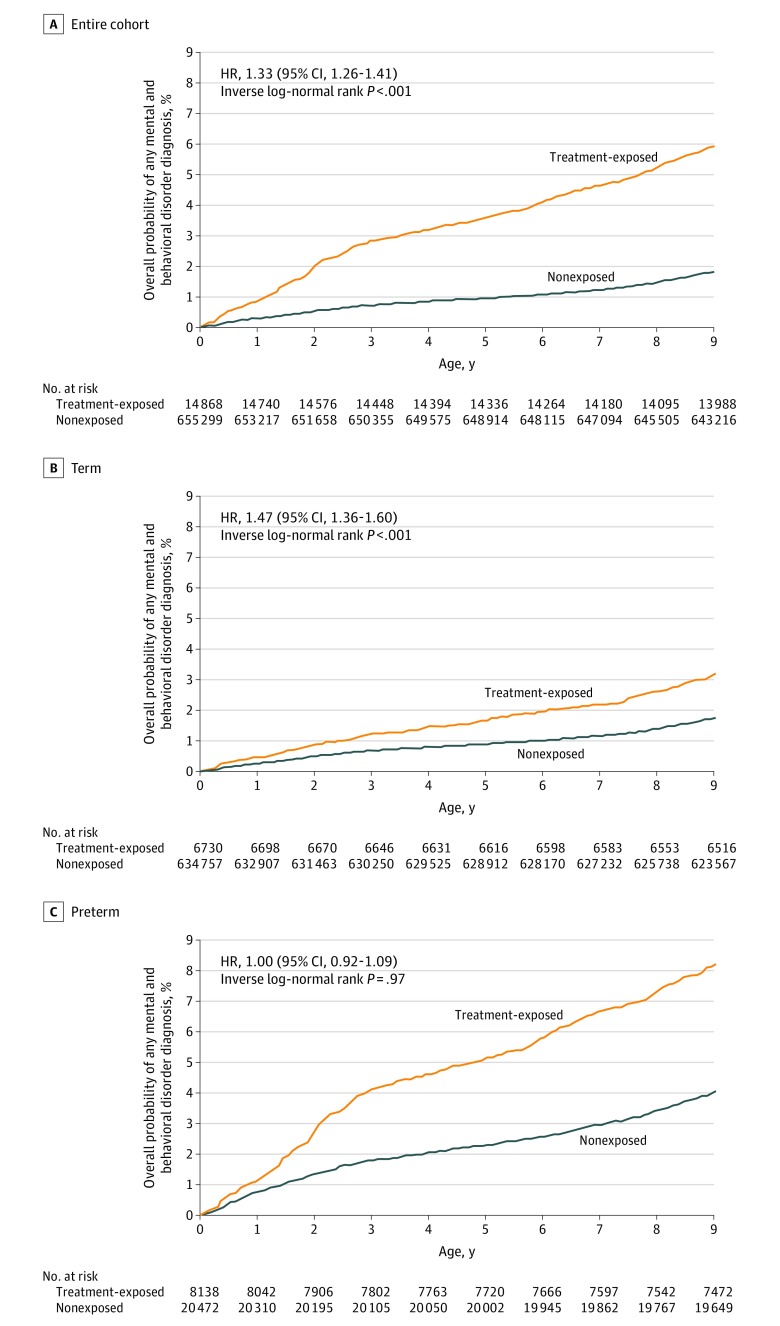
The unadjusted cumulative incidence rates and adjusted HRs from multivariable models (Figure 2; eTable 4 in the Supplement) for any mental and behavioral disorder were statistically significantly higher for the treatment-exposed compared with the nonexposed children in the entire cohort (12.01% vs 6.45%; absolute difference, 5.56% [95% CI, 5.04%-6.19%]; P < .001; HR, 1.33 [95% CI, 1.26-1.41]; P < .001) and in term-born children (8.89% vs 6.31%; absolute difference, 2.58% [95% CI, 1.92%-3.29%]; P < .001; HR, 1.47 [95% CI, 1.36-1.69]; P < .001). In preterm-born children, the cumulative incidence rate of any mental and behavioral disorder was also significantly higher for the treatment-exposed compared with the nonexposed children, but the HR was not significant (14.59% vs 10.71%; absolute difference, 3.38% [95% CI, 2.95%-4.87%]; P < .001; HR, 1.00 [95% CI, 0.92-1.09]; P = .97) (Figure 2). Treatment-exposed children were 1.4 years younger in median age at any first diagnosis than their nonexposed peers (eTable 5 in the Supplement). At the end of follow-up, the probability to remain diagnosis-free for treatment-exposed children was 92.74% in the entire cohort, 95.71% in term-born children, and 90.28% in preterm-born children; corresponding values for nonexposed children were 97.46%, 97.55%, and 94.89% (Figure 3).
Figure 2. Associations Between Maternal Antenatal Corticosteroid Treatment Exposure and Mental and Behavioral Disorders in Children.
aNumber of cases with diagnosis of mental and behavioral disorders and cumulative incidences during follow-up of the entire cohort of children (N = 670 097) and term-born (n = 641 487) and preterm-born (n = 28 610) children eligible for data analyses. See Figure 1 for eligibility criteria for data analyses and definitions of term and preterm births. See eTable 2 in the Supplement for definitions of mental and behavioral disorders. bAbsolute differences may differ from the arithmetic difference of group totals because of rounding. cFrom χ2 statistics. dHazard ratios (HRs), 95% CIs, and P values are from multivariable Cox proportional hazard models adjusting for maternal age at delivery, parity, mode of delivery, maternal smoking during pregnancy, prepregnancy body mass index, premature rupture of membranes (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code O42), gestational diabetes (O24), hypertension in pregnancy (O10, O13-O15), and any lifetime mental disorder diagnosis (F00-F99), child sex, Apgar score (maximum of 1 and 5 minutes), admission to neonatal intensive care unit, weight, and gestational age at birth. eHazard ratios for entire cohort may fall outside the boundaries of HRs for term-born and preterm-born children as a result of differences in the proportion of treatment-exposed and nonexposed children between those born at term and preterm.
Figure 3. Maternal Antenatal Corticosteroid Treatment Exposure and Probability of Any Mental and Behavioral Disorder in Children.
Overall probability of any mental and behavioral disorder diagnosis of treatment-exposed and nonexposed children, from Kaplan-Meier analyses. See Figure 1 for definitions of term and preterm births. See eTable 2 in the Supplement for definition of any mental and behavioral disorder. See eTable 5 in the Supplement for the median age at first diagnosis of any mental and behavioral disorder in the entire cohort of children and in term and preterm children. Hazard ratios (HRs), 95% CIs, and P values are from multivariable Cox proportional hazard models adjusting for maternal age at delivery, parity, mode of delivery, maternal smoking during pregnancy, prepregnancy body mass index, premature rupture of membranes (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] code O42), gestational diabetes (O24), hypertension in pregnancy (O10, O13-O15), and any lifetime mental disorder diagnosis (F00-F99), child sex, Apgar score (maximum at 1 and 5 minutes), admission to neonatal intensive care unit, weight, and gestational age at birth. The age of the oldest child at the last date of follow-up was 11.0 years.
When we excluded children born post-term (n = 30 958, 4.6%) from the analyses, the differences between treatment-exposed and nonexposed children for any disorder were significant in the entire cohort (11.96% vs 6.41%; absolute difference, 5.55% [95% CI, 5.03%-6.10%]; P < .001; HR, 1.29 [95% CI, 1.22-1.37]; P < .001) and in term-born children (8.72% vs 6.27%; absolute difference, 2.46% [95% CI, 1.80%-3.17%]; P < .001; HR, 1.44 [95% CI, 1.33-1.57]; P < .001).
The unadjusted cumulative incidence rates and HRs from multivariable models testing associations between maternal antenatal corticosteroid treatment and the secondary outcomes (the 10 specific mental and behavioral disorders) are shown in Figure 2. In the entire cohort and term-born children, treatment exposure, compared with nonexposure, was significantly associated with psychological development disorders; attention-deficit/hyperactivity or conduct disorders; mixed disorders of conduct and emotions, emotional disorders, disorders of social functioning or tic disorders; other behavioral or emotional disorders; and sleep disorders. In preterm-born treatment-exposed children, compared with nonexposed children, the unadjusted cumulative incidence of mild/moderate/unspecified intellectual disability and sleep disorders were not statistically significantly different, but the adjusted hazard was significantly lower for intellectual disability and higher for sleep disorders.
Within-Sibpair Comparisons
Of 242 240 consecutive sibpairs born at term, 241 621 were eligible for analysis (Figure 1). When within-sibpair differences of all treatment exposure–discordant sibpairs were compared with within-sibpair differences of all nonexposure-concordant sibpairs, the adjusted HR for any mental and behavioral disorder was significantly higher for the treatment-exposed sibling of a discordant sibpair (6.56% vs 4.17% for within-sibpair differences; absolute difference, 2.40% [95% CI, 1.67%-3.21%]; P < .001; HR, 1.38 [95% CI, 1.21-1.58]; P < .001) (eFigure in the Supplement). The HR was also significantly higher for the younger treatment-exposed sibling of a treatment exposure–discordant sibpair, compared with the younger nonexposed sibling of a nonexposure-concordant sibpair (5.74% vs 4.17% for within-sibpair differences; absolute difference, 1.58% [95% CI, 0.66%-2.66%], P < .001; HR, 1.53 [95% CI, 1.29-1.81]; P < .001) (eFigure in the Supplement). The HR was also significantly higher for the treatment-exposed sibling of the first treatment exposure–discordant sibpair for each mother, compared with the first nonexposure-concordant sibpair for each mother (6.90% vs 4.55% for within-sibpair differences; absolute difference, 2.35% [95% CI, 1.51%-3.30%]; P < .001; HR, 1.36 [95% CI, 1.17-1.57]; P < .001) (eFigure in the Supplement).
Discussion
In this population-based retrospective cohort study in which 14 868 children were exposed to maternal antenatal corticosteroid treatment, treatment-exposed children had significantly higher cumulative incidence rates and hazards for any mental and behavioral disorder in a follow-up to 11 years compared with nonexposed children. At the time of the first diagnosis, treatment-exposed children were 1.4 years younger in median age than nonexposed children. These associations were seen in children born at term, and they persisted in within-sibpair comparisons among term-born children, suggesting that unmeasured familial confounding did not explain these associations. However, when preterm-born treatment-exposed children were compared with nonexposed children, the cumulative incidence rate for any mental and behavioral disorder was significantly higher, but the HR from the multivariable model was not statistically significant.
Therefore, antenatal corticosteroid treatment may not pose a risk for mental and behavioral disorders independent of complications and illnesses related to preterm birth. Thus, these findings suggest that while maternal antenatal corticosteroid treatment reduces the risk of neonatal morbidity and mortality, this reduction in short-term risk may be counterbalanced by higher long-term risk for mental and behavioral disorders, at least in children born at term after the treatment exposure. The risk associated with treatment exposure appeared to be comparable in magnitude to the risk of key covariates, such as maternal smoking during pregnancy.
In the entire cohort and in term-born children, the higher unadjusted cumulative incidence rates and hazards were attributable to a wide range of disorders. However, these findings should be regarded as exploratory because of the possibility of type I error.
These findings, especially in term-born children, have several implications. First, while maternal antenatal corticosteroid treatment reduces morbidity and mortality in infants born preterm at less than 34 weeks 0 days,1,2,3,4 the findings call for careful evaluation of the 2016 US guidelines update for treatment extension, namely, recommending treatment when there is a risk for imminent late preterm delivery.1 Treatment in the late preterm window and extension of treatment to women scheduled for cesarean delivery at term before 39 weeks 0 days were recommended in European guidelines between 2016-2019.24 These extensions were no longer included in the 2019 European guidelines update.2 Any extension to treatment indications may lead to substantial increases in treatment-exposed children; compared with infants born before 34 weeks 0 days, the rate of late preterm birth is 2.60-fold greater and of early term birth is 9.42-fold greater.9 Second, while antenatal corticosteroid treatment also reduces risk for respiratory problems beyond 34 weeks,5,6,7,8 these problems are transient and treatable, and no findings, to our knowledge, show longer-term health risks of these newborn adaptation problems.25 However, antenatal corticosteroid treatment may be accompanied by longer-term risks: treatment-exposed neonates of women at risk for late preterm delivery have been shown to have significantly higher risk of hypoglycemia,5,7 which can be associated with a child’s longer-term risk of developmental delay.25 This study extends the longer-term risks of antenatal corticosteroid treatment to childhood mental and behavioral disorders. These disorders are not transient, they tend to track into adulthood,26,27 and they are associated with adverse health, legal, financial, and social problems.28
Third, during the study period, the Finnish guidelines recommended maternal corticosteroid treatment when preterm birth was imminent before 34 weeks 6 days (34 weeks 0 days in the pre-2009 guidelines). Yet 45.27% of the treatment-exposed children were born at term. This number of treatment-exposed term-born children differs from the lower numbers reported in randomized clinical trials5,29 and may reflect that even if the clinical treatment indications do not differ, the eligibility criteria for clinical trials are not necessarily comparable with those applied in everyday clinical practice. Hence, efforts to better predict and prevent preterm delivery are needed.
Limitations
This study has several limitations. First, the observational and sibling-comparison study design limits causal inferences. Second, even though the population-based sample was well characterized, thus allowing multivariable modeling, treatment-exposed and nonexposed pregnancies may have differed in some characteristics that were not measured and residual confounding cannot be ruled out. Third, while the sample was population-based, the homogeneity of the Finnish population precludes generalizations of the findings to different populations.
Fourth, while the sample was large, it still provided limited statistical power to study the outcomes in preterm-born children and preterm-born sibpairs. Fifth, the rates of some of the secondary outcomes were lower than expected, reflecting the relatively young age of the sample and that the disorders were diagnosed in public specialized medical care settings. Sixth, the exact timing of exposure to antenatal corticosteroid treatment and the number and types of treatments given were unknown. One clinical trial has shown that term-born, but not preterm-born, children exposed to multiple courses of antenatal corticosteroids, compared with peers exposed to a single course, displayed worse neurodevelopmental outcomes at 5 years, which included mother-reported executive function and behavior problems.29 Another clinical trial of children born in the 1990s reported no differences in neurodevelopment between children exposed to multiple courses of antenatal corticosteroids and those exposed to a single course plus saline placebo.30
Seventh, while antenatal corticosteroid treatment recorded in the Medical Birth Register and patient case reports showed greater than 97% agreement, approximately 2% of the data were estimated to be incorrectly classified. However, the majority of incorrect classifications were false-negatives, which would be expected to decrease rather than increase ability to detect significant associations. Eighth, while 1333 live-born children who died in infancy were excluded, no individual-level data on deaths after infancy were available. Based on national vital statistics, 0.2 per 1000 are expected to have died after infancy during the follow-up, making the possible effect of this bias small. Ninth, as the studied disorders were diagnosed in specialized medical care settings, this study is likely to miss primary care diagnoses, and findings cannot be generalized to less severe disorders. One study has shown that treatment exposure was associated with mother-reported developmental delay and higher internalizing and externalizing problems in 2- to 5-year-old term-born and preterm-born children,31 suggesting that antenatal corticosteroids may be associated with neurodevelopmental adversities across a range of symptom severity. However, a Cochrane meta-analysis has suggested, based on small-scale randomized clinical trials of children born in the 1970s, that treatment-exposed children had reduced rates of developmental delay but that treatment exposure was not associated with school grades, education, or social and emotional functioning.4
Conclusions
In this population-based cohort study, exposure to maternal antenatal corticosteroid treatment was significantly associated with mental and behavioral disorders in children. These findings may help inform decisions about maternal antenatal corticosteroid treatment.
eTable 1. Agreement Rate Between Maternal Antenatal Corticosteroid Treatment Exposure Recorded in the Medical Birth Register and Patient Case Reports
eTable 2. Definitions of Mental and Behavioral Disorders in Children
eTable 3. Characteristics of Term and Preterm Children According to Maternal Antenatal Corticosteroid Treatment Exposure
eTable 4. Adjusted Hazard Ratios and 95% Confidence Intervals (95% CI) From Multivariable Models Testing Association Between Maternal Antenatal Corticosteroid Treatment and Covariates With Any Mental and Behavioral Disorder in the Entire Cohort of Children and in Term (≥ 37 Gestational Weeks) and in Preterm (< 37 Gestational Weeks) Children
eTable 5. Median Ages (Interquartile Ranges) in Years at First Diagnosis of Mental and Behavioral Disorders in the Entire Cohort of Children and in Term (≥ 37 Gestational Weeks) and in Preterm (< 37 Gestational Weeks) Children According to Maternal Antenatal Corticosteroid Treatment Exposure
eFigure. Associations Between Maternal Antenatal Corticosteroid Treatment Exposure and Mental and Behavioral Disorders in Term Maternal Sibling Pairs
References
- 1.Committee on Obstetric Practice Committee Opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130(2):e102-e109. doi: 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 2.Sweet DG, Carnielli V, Greisen G, et al. . European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology. 2019;115(4):432-450. doi: 10.1159/000499361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO recommendations on interventions to improve preterm birth outcomes. World Health Organization. Published November 2015. Accessed April 9, 2020. https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/preterm-birth-guideline/en/ [PubMed]
- 4.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. doi: 10.1002/14651858.CD004454.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. ; NICHD Maternal–Fetal Medicine Units Network . Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311-1320. doi: 10.1056/NEJMoa1516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stutchfield P, Whitaker R, Russell I; Antenatal Steroids for Term Elective Caesarean Section (ASTECS) Research Team . Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331(7518):662. doi: 10.1136/bmj.38547.416493.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355(15044):i5044. doi: 10.1136/bmj.i5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JPA. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Cochrane Database Syst Rev. 2009;4(4):CD006614. doi: 10.1002/14651858.CD006614.pub2 [DOI] [PubMed] [Google Scholar]
- 9.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep. 2018;67(8):1-50. [PubMed] [Google Scholar]
- 10.Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: what do we do now? Am J Obstet Gynecol. 2016;215(4):423-430. doi: 10.1016/j.ajog.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Harding JE. Antenatal glucocorticoids for late preterm birth? N Engl J Med. 2016;374(14):1376-1377. doi: 10.1056/NEJMe1601867 [DOI] [PubMed] [Google Scholar]
- 12.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63-84. doi: 10.1196/annals.1314.006 [DOI] [PubMed] [Google Scholar]
- 13.Reynolds RM, Seckl JR. Antenatal glucocorticoid treatment: are we doing harm to term babies? J Clin Endocrinol Metab. 2012;97(10):3457-3459. doi: 10.1210/jc.2012-3201 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez A, Wang Y, Ali Khan A, Cartwright R, Gissler M, Järvelin M-R. Antenatal corticosteroid therapy (ACT) and size at birth: a population-based analysis using the Finnish Medical Birth Register. PLoS Med. 2019;16(2):e1002746. doi: 10.1371/journal.pmed.1002746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gissler M. Registration of births and induced abortions in the Nordic countries. Yearb Popul Res Finl. 2010;45:171-178. [Google Scholar]
- 16.Finnish Medical Society Duodecim Current Care Guidelines website. Accessed April 17, 2020. https://www.kaypahoito.fi
- 17.Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40(6):505-515. doi: 10.1177/1403494812456637 [DOI] [PubMed] [Google Scholar]
- 18.Lampi KM, Sourander A, Gissler M, et al. . Brief report: validity of Finnish registry-based diagnoses of autism with the ADI-R. Acta Paediatr. 2010;99(9):1425-1428. doi: 10.1111/j.1651-2227.2010.01835.x [DOI] [PubMed] [Google Scholar]
- 19.Kong L, Norstedt G, Schalling M, Gissler M, Lavebratt C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics. 2018;142(3):e20180776. doi: 10.1542/peds.2018-0776 [DOI] [PubMed] [Google Scholar]
- 20.Pyhälä R, Wolford E, Kautiainen H, et al. . Self-reported mental health problems among adults born preterm: a meta-analysis. Pediatrics. 2017;139(4):20162690. doi: 10.1542/peds.2016-2690 [DOI] [PubMed] [Google Scholar]
- 21.Heinonen K, Kajantie E, Pesonen A-K, et al. . Common mental disorders in young adults born late-preterm. Psychol Med. 2016;46(10):2227-2238. doi: 10.1017/S0033291716000830 [DOI] [PubMed] [Google Scholar]
- 22.Lahti M, Eriksson JG, Heinonen K, et al. . Late preterm birth, post-term birth, and abnormal fetal growth as risk factors for severe mental disorders from early to late adulthood. Psychol Med. 2015;45(5):985-999. doi: 10.1017/S0033291714001998 [DOI] [PubMed] [Google Scholar]
- 23.Sudan M, Kheifets LI, Arah OA, Divan HA, Olsen J. Complexities of sibling analysis when exposures and outcomes change with time and birth order. J Expo Sci Environ Epidemiol. 2014;24(5):482-488. doi: 10.1038/jes.2013.56 [DOI] [PubMed] [Google Scholar]
- 24.Sweet DG, Carnielli V, Greisen G, et al. . European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 Update. Neonatology. 2017;111(2):107-125. doi: 10.1159/000448985 [DOI] [PubMed] [Google Scholar]
- 25.Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics. 2012;130(2):e265-e272. doi: 10.1542/peds.2012-0079 [DOI] [PubMed] [Google Scholar]
- 26.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837-844. doi: 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- 27.Copeland WE, Shanahan L, Costello EJ, Angold A. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch Gen Psychiatry. 2009;66(7):764-772. doi: 10.1001/archgenpsychiatry.2009.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copeland WE, Wolke D, Shanahan L, Costello EJ. Adult functional outcomes of common childhood psychiatric problems: a prospective, longitudinal study. JAMA Psychiatry. 2015;72(9):892-899. doi: 10.1001/jamapsychiatry.2015.0730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asztalos E, Willan A, Murphy K, et al. ; MACS-5 Collaborative Group . Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5). BMC Pregnancy Childbirth. 2014;14:272. doi: 10.1186/1471-2393-14-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowther CA, Anderson PJ, McKinlay CJD, et al. ; ACTORDS Follow-up Group . Mid-childhood outcomes of repeat antenatal corticosteroids: a randomized controlled trial. Pediatrics. 2016;138(4):e20160947. doi: 10.1542/peds.2016-0947 [DOI] [PubMed] [Google Scholar]
- 31.Wolford E, Lahti-Pulkkinen M, Girchenko P, et al. . Associations of antenatal glucocorticoid exposure with mental health in children. Psychol Med. 2020;50(2):247-257. doi: 10.1017/S0033291718004129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Agreement Rate Between Maternal Antenatal Corticosteroid Treatment Exposure Recorded in the Medical Birth Register and Patient Case Reports
eTable 2. Definitions of Mental and Behavioral Disorders in Children
eTable 3. Characteristics of Term and Preterm Children According to Maternal Antenatal Corticosteroid Treatment Exposure
eTable 4. Adjusted Hazard Ratios and 95% Confidence Intervals (95% CI) From Multivariable Models Testing Association Between Maternal Antenatal Corticosteroid Treatment and Covariates With Any Mental and Behavioral Disorder in the Entire Cohort of Children and in Term (≥ 37 Gestational Weeks) and in Preterm (< 37 Gestational Weeks) Children
eTable 5. Median Ages (Interquartile Ranges) in Years at First Diagnosis of Mental and Behavioral Disorders in the Entire Cohort of Children and in Term (≥ 37 Gestational Weeks) and in Preterm (< 37 Gestational Weeks) Children According to Maternal Antenatal Corticosteroid Treatment Exposure
eFigure. Associations Between Maternal Antenatal Corticosteroid Treatment Exposure and Mental and Behavioral Disorders in Term Maternal Sibling Pairs