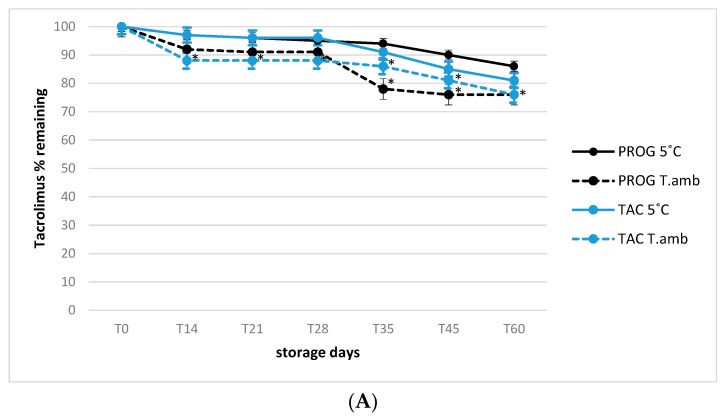
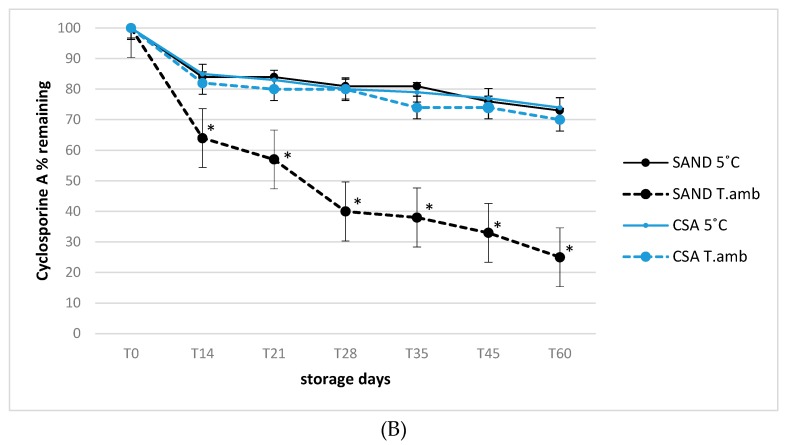
Figure 2.
(A) Active principle stability in 0.1% tacrolimus (A) eye drops formulations stored at 5 °C and 25 °C (T.amb) under user simulated conditions (see Experimental for details); * p < 0.05, T.amb versus 5 °C storage temperature. (B) Active principle stability in 1.0% cyclosporine A (B) eye drops formulations stored at 5 °C and 25 °C (T.amb) under user simulated conditions (see Experimental for details); * p < 0.05, T.amb versus 5 °C storage temperature.