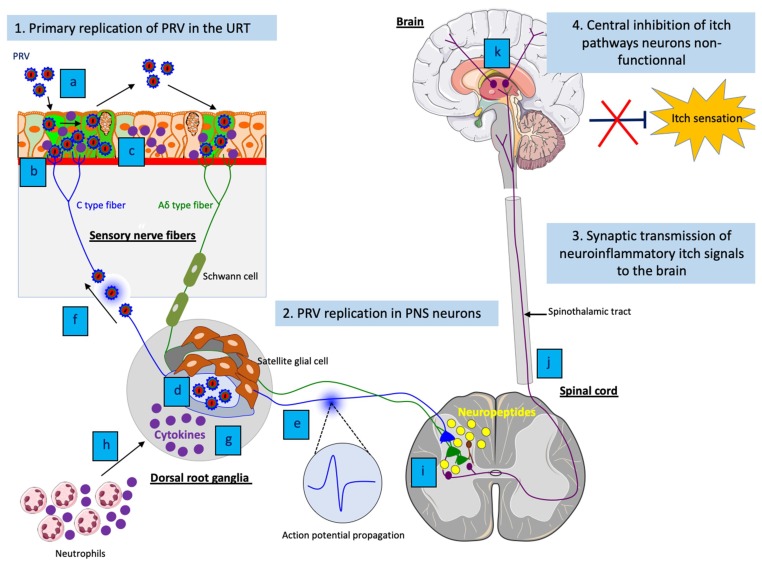
Figure 3.
Model of PRV-induced neuropathic itch in non-natural hosts. (1) (a) Efficient viral replication and spread of PRV in the respiratory epithelium; (b) PRV particles activate axons terminals of sensory PNS neurons; (c) Activated sensory PNS neurons trigger inflammatory signaling pathways and produce pro-inflammatory cytokines that are released in PNS neurons and locally at axon terminals. (2) (d) Efficient PRV replication in cell bodies of PNS neurons and release of new progeny virions; (e) Spontaneous hyperexcitability of neurons and increase of action potential firing as well as (f) reseeding of new progeny virions back to the epithelium increases electrical coupling of axons and contributes to the pruritus; (b) Amplification of the inflammatory response in PNS neurons; (h) The production of pro-inflammatory cytokines in PNS neurons attract neutrophils and other immune cells to the site of infection and propagate the neuroinflammation. (3) (i) Release of neuropeptides from activated PNS neurons stimulate excitative interneurons in the dorsal horn of the spinal cord; (j) The excitation spreads to spinothalamic tract neurons, which in turn send neuroinflammatory itch signals to the brain; (k) These signals are relayed and processed in the somatosensory cortex. (4) The central itch inhibition pathways are likely dampened or disabled, resulting in an unstoppable pruritus.