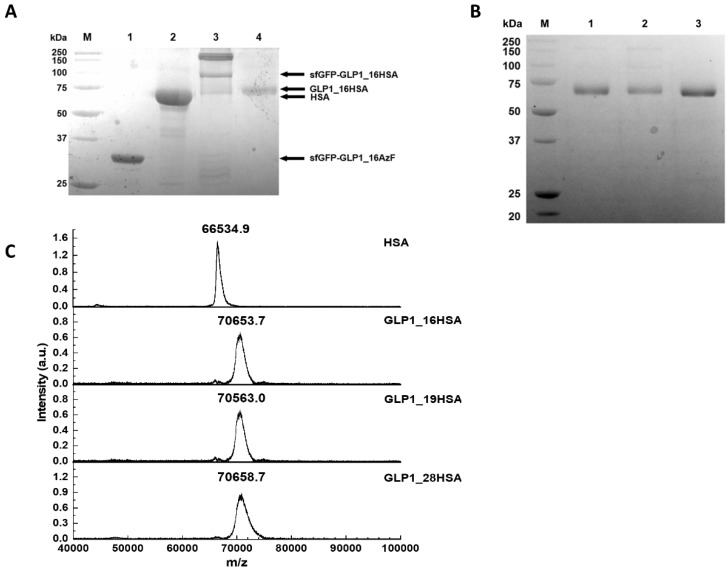
Figure 5.
Confirmation of GLP1_HSA variants and their intermediates during production. (A) Image of the Coomassie Brilliant Blue-stained gel indicating the presence of the protein intermediates of GLP1_HSA production. GLP1_16HSA is presented as a representative of the GLP1_HSA variants. Protein molecular weight standards (lane M), sfGFP-GLP1_16AzF (lane 1), purified HSA (lane 2), sfGFP-GLP1_16HSA (lane 3), and GLP1_16HSA (lane 4). In lane 3, the bands between 150 and 250 kDa are attributable to aggregates. (B) Coomassie Brilliant Blue-stained protein gel of the purified GLP1_16HSA (lane 1), GLP1_19HSA (lane 2), and GLP1_28HSA (lane 3). Protein molecular weight standards are shown in lane M. (C) Matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS) analysis of GLP1_HSA variants to determine their intact masses (a.u. indicates arbitrary unit).