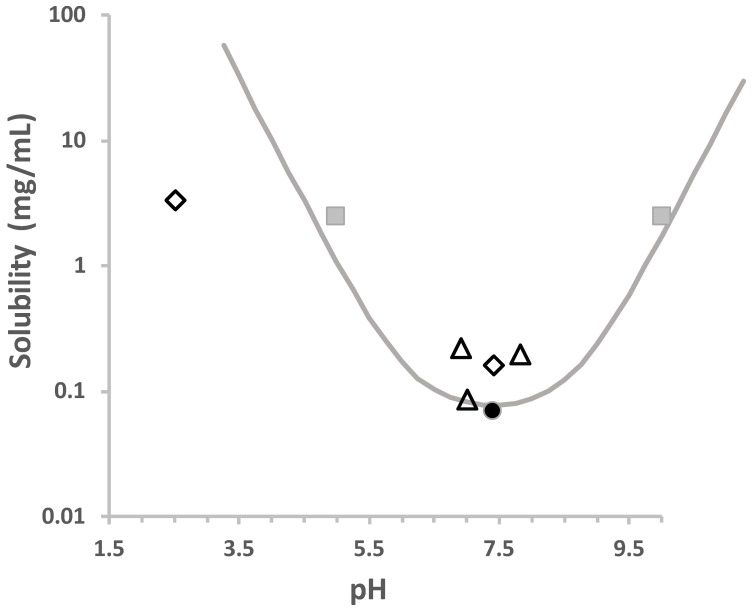
Figure 3.
Effect of pH on CIP aqueous solubility calculated using the following equation: , with pka1 = 6.2, pka2 = 8.6 [20,75]. Individual values are from Yu et al. [76] (empty triangles); McShane et al. [20] (plain dot); Blokhina et al. [77] (empty diamond; Roca Jalil et al. [78] (square). Values were measured between 25 and 30 °C.