Table 3.
PI3K/HDAC Enzyme Inhibitory Activities of 5-Substituted Quinazolinones with Purine Hinge Binding Groupa
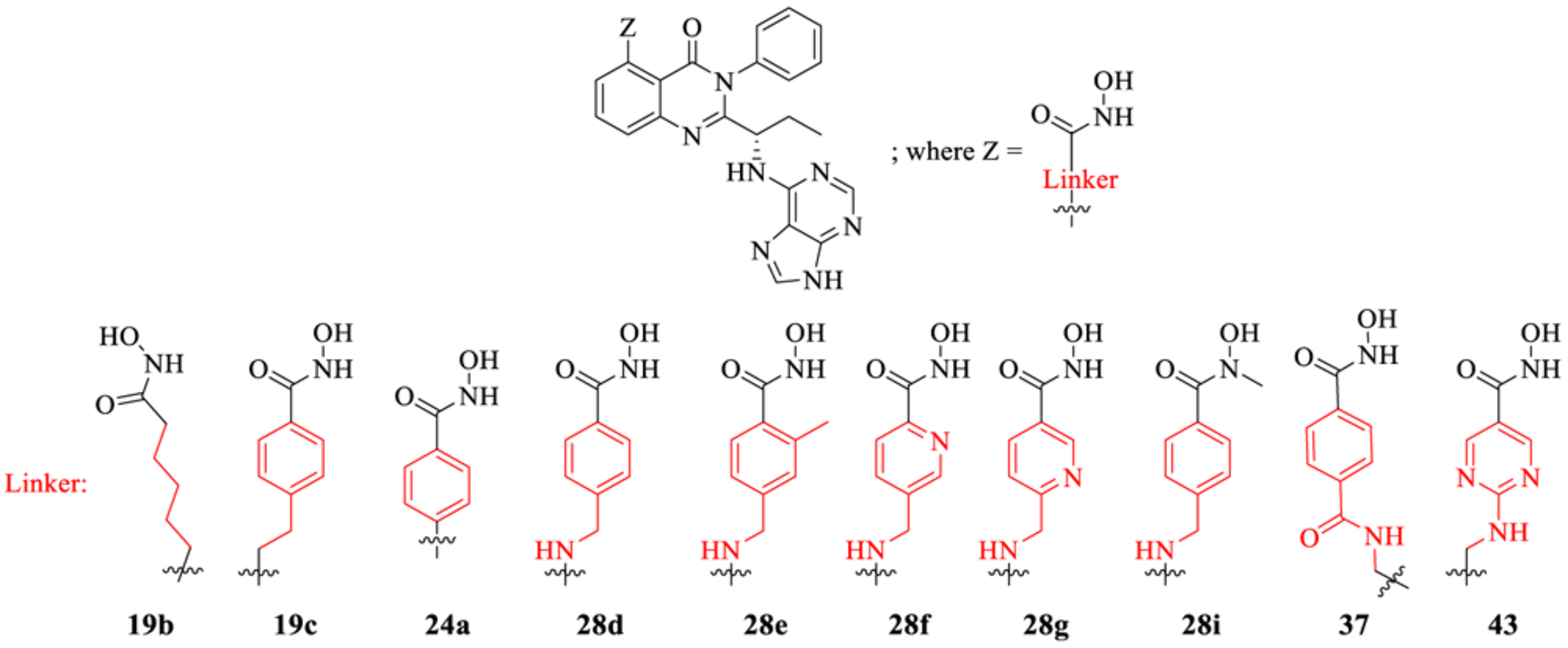 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | |||||||||||||||
| PI3K | HDAC | ||||||||||||||
| compd | α | β | γ | δ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 19b | 122 | 286 | 43 | 0.2 | 1390 | 3030 | 2400 | 8060 | 13 | 856 | 1240 | 1840 | |||
| 19c | NT | NT | NT | 5 | >105 | 889 | 3840 | 9000 | |||||||
| 24a | NT | NT | NT | 0.7 | NT | NT | NT | NT | NT | 362 | NT | 71 | NT | NT | NT |
| 28d | 254 | 103 | 47 | 3925 | 7173 | 6699 | 1751 | 2144 | 5 | 229 | 250 | 3335 | 14700 | 324 | |
| 28e | 517 | 203 | 41 | NT | NT | 2699 | 6922 | NT | NT | ||||||
| 28f | NT | NT | NT | 43 | NT | NT | NT | NT | NT | 169 | NT | NT | NT | NT | NT |
| 28g | 392 | 135 | 26 | 6067 | 10990 | 381 | 439 | 9 | 104 | 204 | 922 | 9996 | 2255 | ||
| 28i | 387 | 162 | 81 | ||||||||||||
| 37 | 266 | 234 | 0.6 | 8434 | 10 | 23120 | 138 | 19890 | 1778 | ||||||
| 43 | 422 | 995 | 49 | 67870 | 25480 | 1428 | 3827 | 6 | 60 | 320 | 5349 | ||||
| PI-103 | 4 | 7 | 81 | 8 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| TSA | NT | NT | NT | NT | 10 | 34 | 19 | NT | NT | 2 | NT | 445 | NT | 45 | 4826 |
| TMP 269 | NT | NT | NT | NT | NT | NT | NT | 244 | 278 | NT | 62 | NT | 19 | NT | NT |
Compounds were tested in singlet 10-dose IC50 mode with 3-fold serial dilution starting at 1 μM for PI3Kα, β, γ, δ, and 10 μM for HDAC1–11 enzymes. Empty cells indicate no inhibition or compound activity that could not be fit to an IC50 curve. NT = Compound not tested against enzyme. PI-103 was used as a control compound for PI3Ks, whereas trichostatin A (TSA) and TMP 269 were used as control compounds for HDAC enzymes.
