Figure 1.
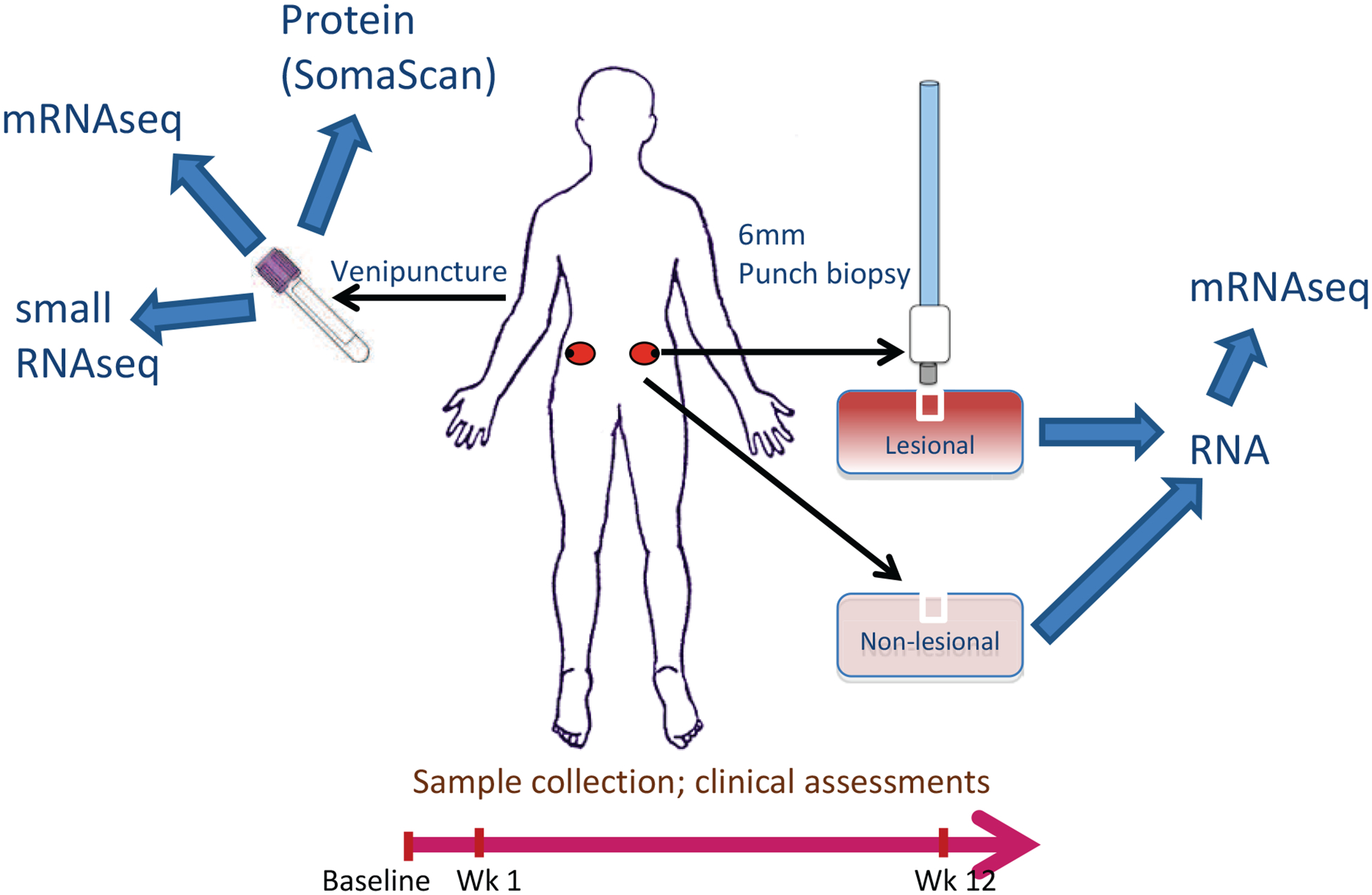
Study Overview
Participants were assessed at baseline, week one and week 12 of therapy. Participant sampling comprised blood testing, urine collection, lesional and non-lesional skin biopsies (from photoprotected sites on the lower back/buttock, from the edge of plaques and at a minimum distance from previous biopsy sites). RNA-Seq was conducted on mRNA from blood, lesional and non-lesional skin and miRNA from blood. Proteomic assessment was conducted on serum.
