Figure 4.
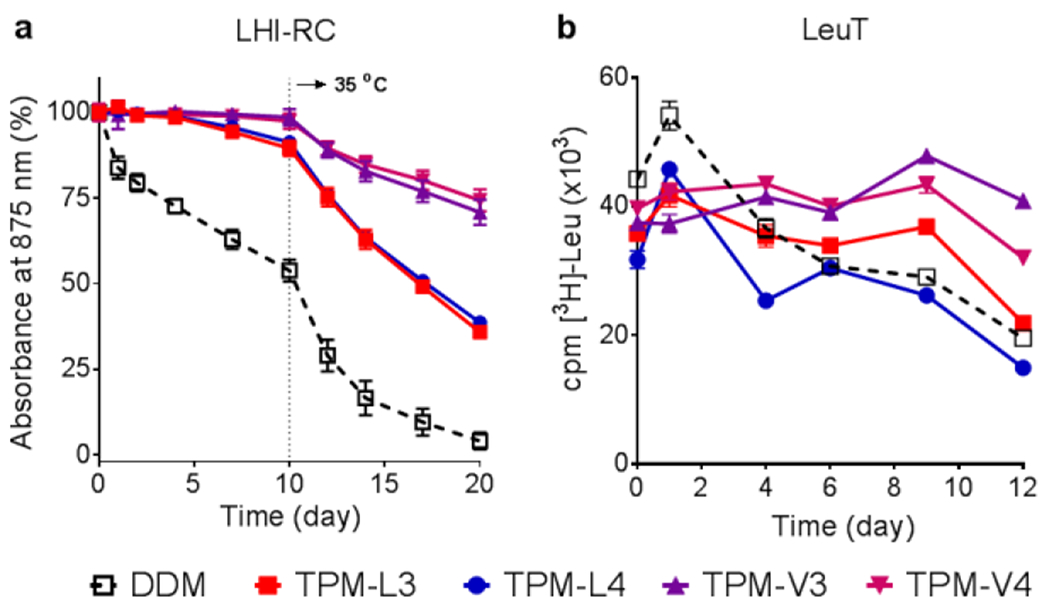
Time course stability of (a) LHI-RC complex (b) LeuT solubilized in novel agents (TPM-L3, TPM-L4, TPM-V3 and TPM-V4). A conventional detergent, DDM, was used as control. LHI-RC and LeuT stability assays were carried out at detergent concentrations of CMC+0.05 wt% and CMCs+0.04 wt%, respectively. LHI-RC stability was assessed by monitoring the absorbance of the complexes at 875 nm (A875) at regular intervals during a 20-day incubation. The samples were stored at room temperature for the first 10-day incubation and the incubation temperature was increased and maintained at 35 °C for the next 10 days. Error bars, SEM, n = 2. LeuT stability was assessed by measuring the ability of the transporter to bind the radio-labelled substrate (3[H]-leucine (Leu)) via scintillation proximity assay (SPA) and was monitored at regular intervals over the course of a 12-day incubation at room temperature. Error bars, SEM, n = 2–3.
