Scheme 1.
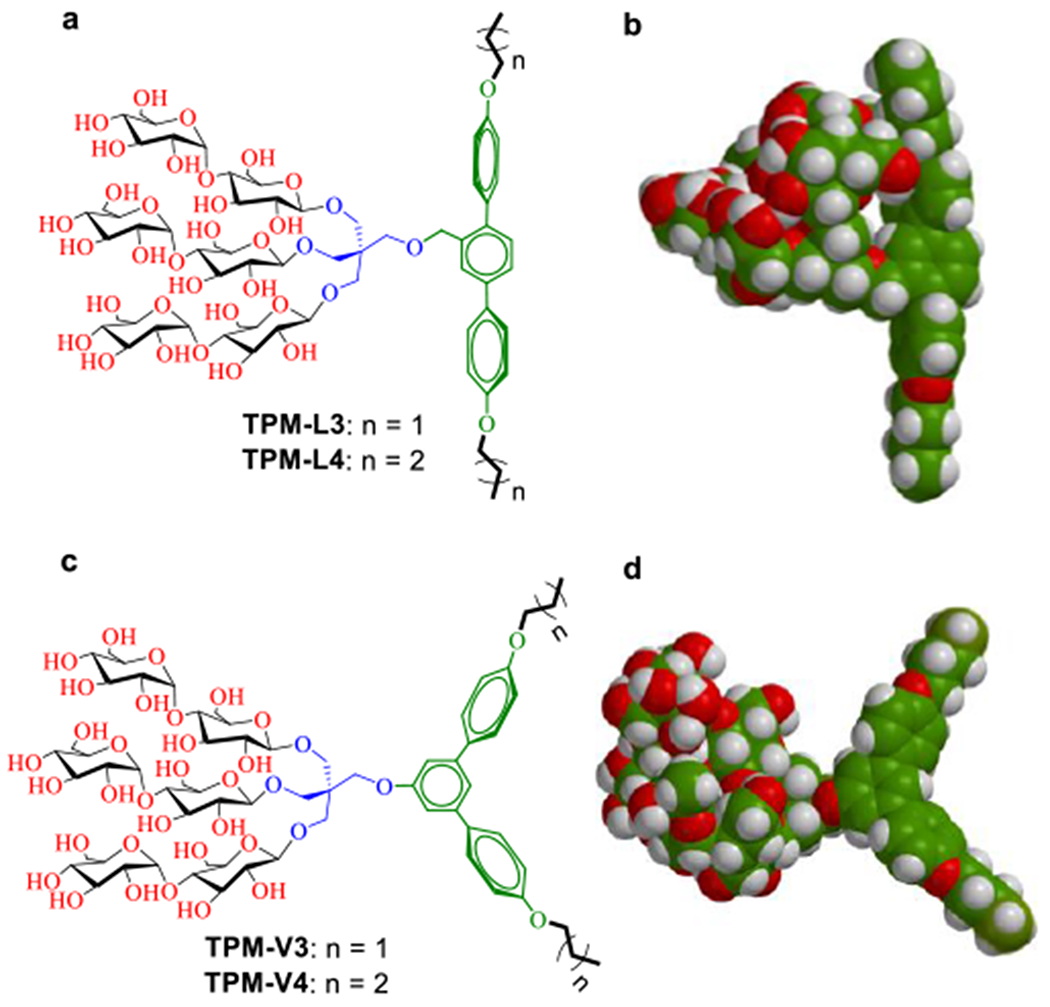
(a,c) Chemical structures of terphenyl-cored maltosides (TPMs) and (b,d) space-filing models for the energy-minimized structures of TPM-L4 and TPM-V4. These amphiphiles commonly contain a trimaltoside head group connected to the lipophilic group using a neopentyl glycol linker. The lipophilic group features with three consecutive phenyl rings (i.e., terphenyl group) with alkyl chain appendages at both terminals. The three phenyl rings are organized in a linear or in a bent arrangement (V-shaped), and are thus designated TPM-Ls (a,b) and TPM-Vs (c,d), respectively. Two short alkyl chains (propyl (C3) and butyl (C4)) were introduced as the terminal units, as indicated in the detergent designation. The energy-minimized structures of TPM-L4 and TPM-V4 as obtained by DFT calculations (B3LYP/6–31G* level) in water (model, space filling). Carbon, hydrogen and oxygen are indicated in green, white and red, respectively.
