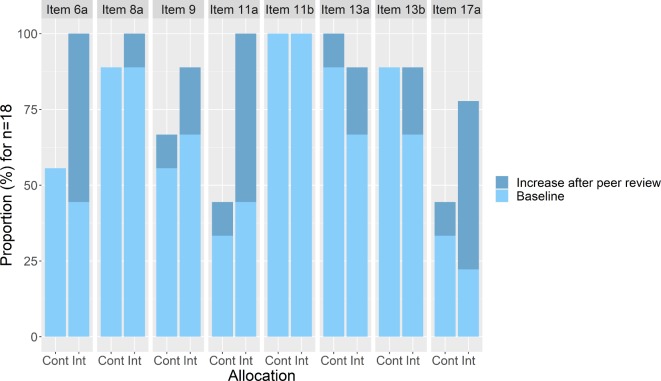
Figure 4.
Proportion of manuscripts (n=18) where each CONSORT item is adequately reported. CONSORT items: 6a: ‘Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed’; 8a: ‘Method used to generate the random allocation sequence’; 9: ‘Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned’; 11a: ‘If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how’); 11b: ‘If relevant, description of the similarity of interventions’; 13a: ‘For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome’; 13b: ‘For each group, losses and exclusions after randomisation, together with reasons’; 17a: ‘For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval)’). CONSORT, Consolidated Standards of Reporting Trials; Cont, control group; Int, intervention group.