Fig. 5. Summary of the mechanism of substrate translocation in Lon protease.
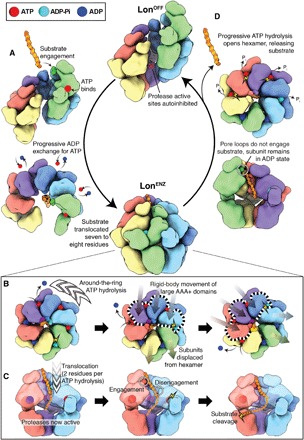
(A) In the absence of substrate, Lon that is fully ADP-bound adopts a left-handed, open lock washer configuration with autoinhibited protease active sites. Simultaneous ATP and substrate binding to the uppermost protomer initiates the reorganization toward the proteolytically active LonENZ conformer. Subunits then progressively exchange ADP for ATP in a counterclockwise manner. The LonENZ conformer is achieved upon binding a total of four ATP molecules, finalized with one hydrolysis event to close the ring. Hydrolysis occurs in the subunit that had previously been at the top of the spiral (green) when it reaches the lowest position of the ATPase spiral within the closed hexamer. The rearrangement results in a seven to eight-residue translocation of the substrate peptide, which is now threaded through the center of the central ATPase channel. (B) The LonENZ conformation is competent for hand-over-hand translocation, and the protease domains form an enclosed sixfold symmetric ring and with active protease sites. Around-the-ring ATP hydrolysis drives substrate translocation, with the pore loops of the ATP-bound subunits engaging with substrate (substrate-interacting pore loops shown using space-filling representation). ATP binding and hydrolysis at opposite sides of the ring result in rigid-body movements of the three upper-most ATP-bound subunits (dotted outline), leading to two-residue translocation steps toward the protease. (C) Cutaway view showing progression of substrate along the central axis, being translocated two amino acids per hydrolysis event, and substrate binding within the active site for cleavage. As in (B), substrate-interacting pore loops shown using space-filling representation. Substrate is directed to the protease, where it can be positioned for cleavage. A summary of the LonENZ mechanochemical cycle is shown in movie S1. (D) Inability of the pore loop 1 of an ADP-bound subunit to bind substrate either due to reaching the terminus of a substrate or encountering a tightly folded domain will trigger a return to the LonOFF conformation. The remaining ATP- and substrate-bound subunits will continue around-the-ring hydrolysis and translocation, but each will remain in the ADP-bound conformation after nucleotide hydrolysis. Remaining nonproteolyzed substrate peptides are released. A summary of the entire conformational cycle is shown in movie S3.
