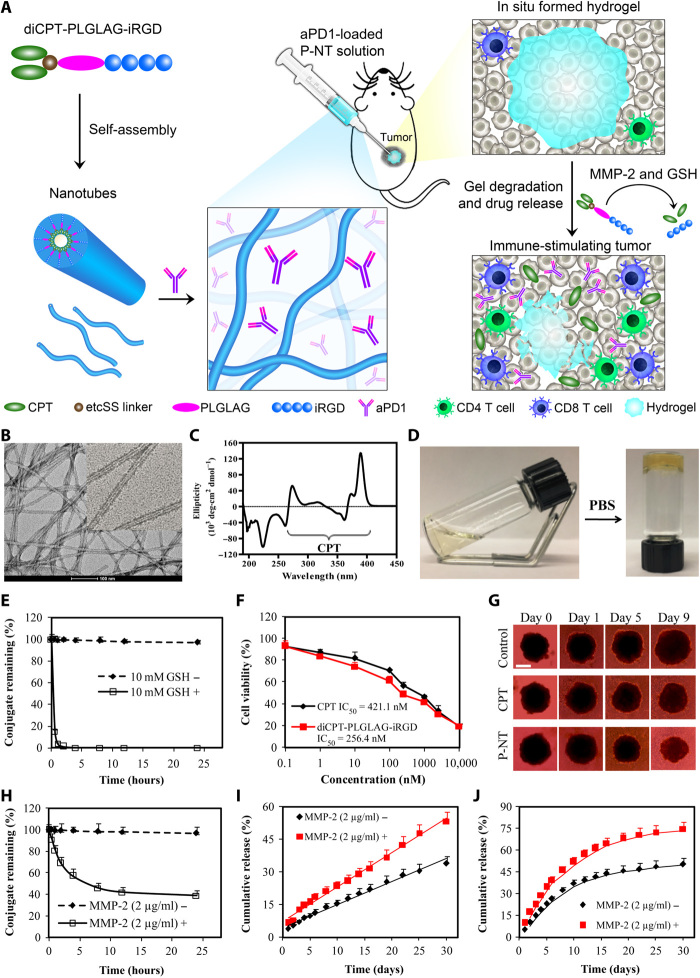
Fig. 1. Schematic and characterization of in situ formed P-NT–aPD1 hydrogel.
(A) Schematic illustration of localized CPT and aPD1 delivery using an in situ formed supramolecular hydrogel to attain bioresponsive drug release and tumor microenvironment regulation. (B) Representative transmission electron microscopy (TEM) images of diCPT-PLGLAG-iRGD nanotubes (P-NT). Scale bar, 100 nm. (C) The circular dichroism (CD) spectrum of the diCPT-PLGLAG-iRGD nanotubes solution. (D) Photographs of the sol-gel transition of P-NT upon the addition of phosphate-buffered saline (PBS). (E) Degradation profiles of diCPT-PLGLAG-iRGD in the presence or absence of glutathione (GSH) (10 mM). Data are given as means ± SD (n = 3). (F) In vitro cytotoxicity studies of free CPT and diCPT-PLGLAG-iRGD toward GL-261 brain cancer cells. IC50, median inhibitory concentration. (G) Inhibition of tumor spheroid growth was evaluated following treatment with free CPT or P-NT. Spheroids treated with drug-free Dulbecco’s modified Eagle’s medium were used as the blank control. Scale bar, 500 μm. (H) The degradation profiles of 200 μM diCPT-PLGLAG-iRGD solutions incubated in the presence or absence of matrix metalloproteinase 2 (MMP-2; 2 μg/ml). Data are given as means ± SD (n = 3). (I) Cumulative release profiles of CPT prodrugs (including diCPT-PLGLAG-iRGD and diCPT-PLG) and (J) aPD1 from P-NT–aPD1 hydrogels incubated in PBS with or without MMP-2. Data are given as means ± SD (n = 3). Photo credit: Feihu Wang, Johns Hopkins University.