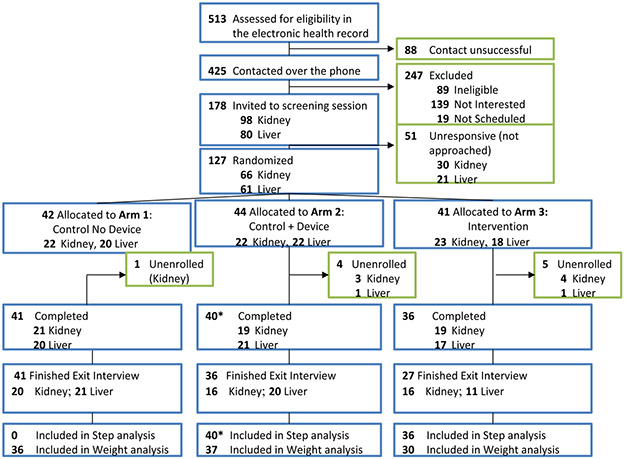
Figure 1.
Study flow diagram
* 1 patient died 10 days prior to study completion, steps were included in analysis. Patients in the Control No Device arm did not have measured steps. The Control + Device arm included an accelerometer to measure daily steps. The Intervention arm included an accelerometer, daily step goal targets with loss-framed financial incentives, and biweekly text messages with health engagement questions.