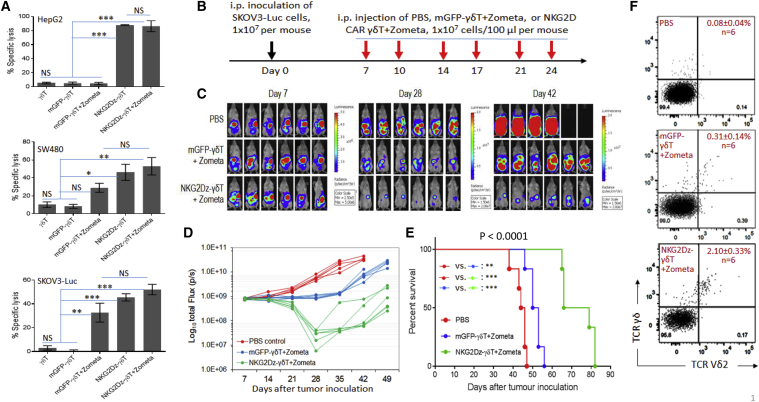
Figure 5.
Zometa Treatment and Effects of Vγ9Vδ2 T Cells Expressing NKG2D CAR (NKG2Dz)
(A) In vitro cytolytic effects on Zometa-treated tumor cells. Target tumor cells tested were HepG2 human hepatocarcinoma cells, SW480 human colon carcinoma cells, and SKOV3 human ovarian cancer cells. Tumor cells were either pre-treated with 1 μM Zometa (+Zometa) or without Zometa treatment. Effector cells tested include Vγ9Vδ2 T cells (γδ T), γδ T electroporated with mGFP CAR, and γδ T electroporated with NKG2Dz RNA CAR. Delfia EuTDA cytotoxicity assay (3 h EuTDA culturing) was used to assess tumor cell lysis efficiency at an effector to target ratio of 10:1. The results of one representative experiment out of three are shown. Data shown are mean ± SD of triplicates. (B) Experimental outline of the animal study. NSG mice (n = 6 per group) were i.p. injected with the SKOV3-Luc human ovarian cancer cells (1 × 107 per mouse). The treatment started 7 days after tumor cell inoculation, twice a week for 3 weeks. Zometa (1 mg/kg) was i.p. injected 1 day before cell injection, followed by 1 × 107 Vγ9Vδ2 T cells per i.p. injection 1 day later. The mice were followed with serial weekly imaging to assess the tumor burden. (C–F) In vivo effects. (C) Tumor burden images by bioluminescent imaging (BLI) on days 7, 28, and 42. (D) Quantitative analysis of SKOV3-luc cell bioluminescence signals obtained in the in vivo experiment. Tumor burden over time by BLI is shown. Each mouse is represented by one line. (E) Kaplan-Meier analysis of survival of the in vivo experiment. Statistical analysis of survival between groups was performed using the log-rank test. (F) Blood human Vγ9Vδ2 T cells. Mouse blood samples were collected 7 days after the last CAR-T cell injection for flow cytometric analysis. Examples of anti-human TCR Pan γδ antibody and anti-human TCR Vδ2 antibody double staining from each group with mean percentages (SD) of human Vγ9Vδ2 T cells are shown. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS, not significant.