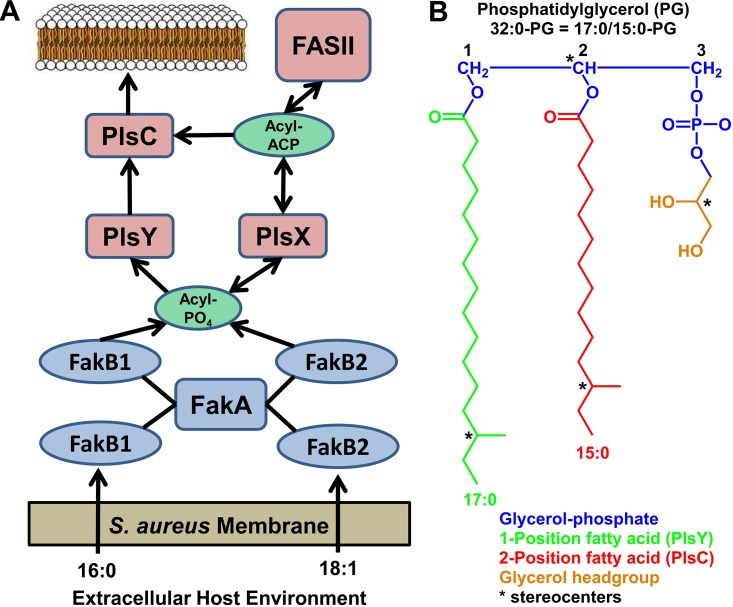
FIG 1.
Model for the utilization of host FAs for phosphatidylglycerol (PG) synthesis by S. aureus. (A) FA kinase is the only pathway for the activation of extracellular FAs in S. aureus. Host FAs bind to one of the two FA binding proteins. FakB1 specifically binds saturated FA (16:0), and FakB2 selectively binds monounsaturated FA (18:1). The FakB(FA) complexes are phosphorylated by FakA, converting host FAs to the key intermediate, acyl-PO4. The resulting acyl-PO4 has two fates: PlsX and PlsY. PlsX converts acyl-PO4 to acyl-ACP that is elongated by FASII before being converted again to acyl-PO4 by PlsX. FASII produces 15:0 ACP that is preferentially used by PlsC to acylate the 2 position. (B) Structure of PG. The major membrane phospholipid of S. aureus consists of a glycerol phosphate backbone (blue) that is first acylated in the 1 position by acyl-PO4-dependent PlsY (green) followed by acylation of the 2 position by 15:0-ACP-selective PlsC (red). The glycerol headgroup (gold) is added by a series of three enzymes. There are four stereocenters (*) in the example show the abundant 17:0/15:0 PG molecular species (32:0 PG; m/z 721) of S. aureus.