Figure 2. Risks of the Primary and Key Secondary End Points in Patients With Recent and Remote Myocardial Infarction (MI) Randomized to Placebo vs Evolocumab.
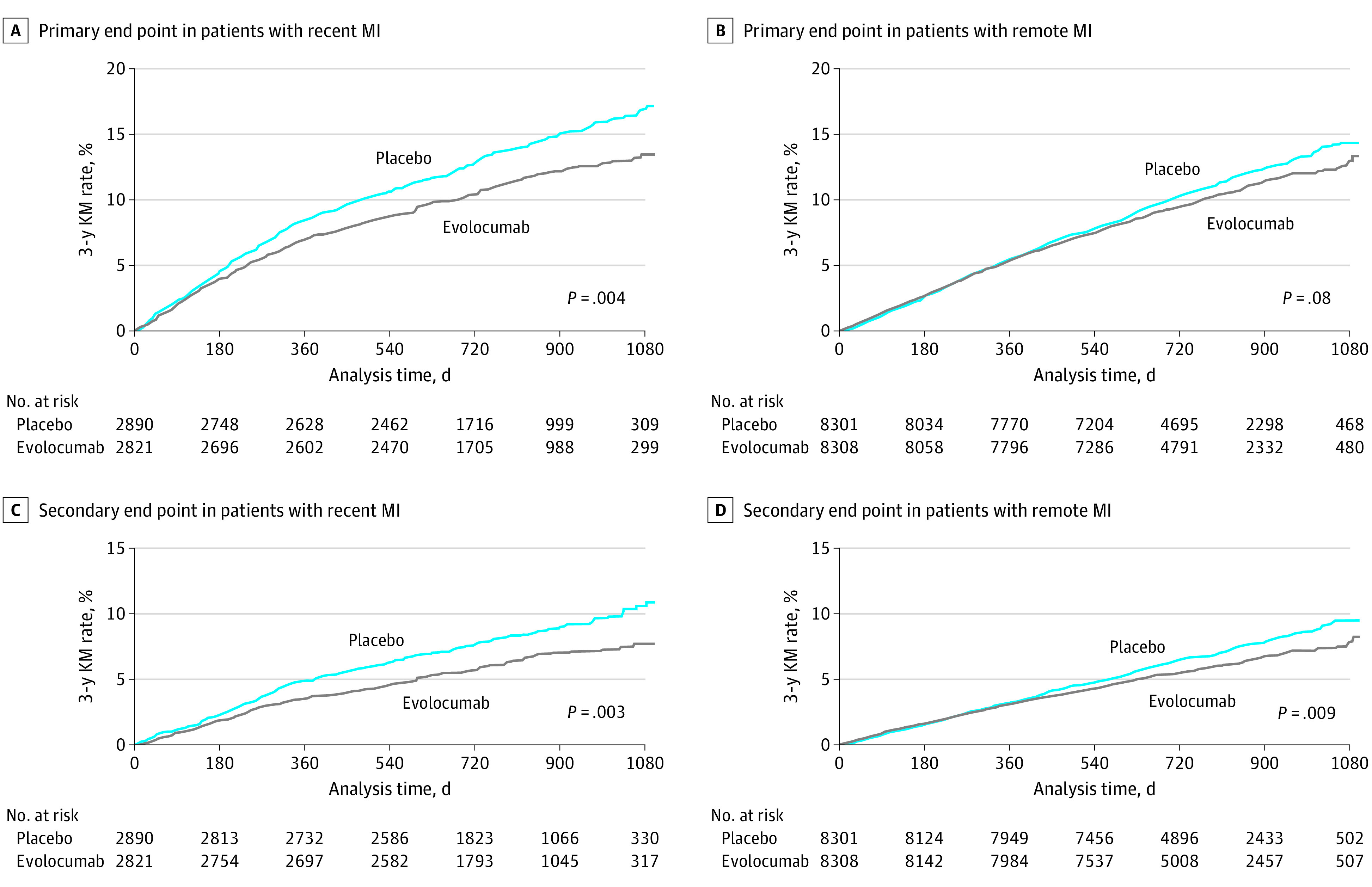
A, Primary end point among patients with recent MI randomized to placebo vs evolocumab (hazard ratio, 0.81; 95% CI, 0.70-0.93). The absolute risk reduction was 3.7% (95% CI, 1.3-6.1), and the number needed to treat over 3 years was 27. B, Primary end point among patients with remote MI randomized to placebo vs evolocumab (hazard ratio, 0.92; 95% CI, 0.84-1.01). The absolute risk reduction was 1.1% (95% CI, −0.6 to 2.7), and the number needed to treat over 3 years was 91. C, Key secondary end point among patients with recent MI randomized to placebo vs evolocumab (hazard ratio, 0.75; 95% CI, 0.62-0.91). The absolute risk reduction was 3.2% (95% CI, 1.2-5.2), and the number needed to treat over 3 years was 31. D, Key secondary end point among patients with remote MI randomized to placebo vs evolocumab (hazard ratio, 0.85; 95% CI, 0.76-0.96). The absolute risk reduction was 1.3% (95% CI, −0.1 to 2.7), and the number needed to treat over 3 years was 79. A total of 5711 patients had recent MI (ie, 1 to 12 months prior to randomization) and 16 609 patients had remote MI (ie, more than 12 months prior to randomization). The primary composite end point was cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. The key secondary end point was cardiovascular death, MI, or stroke. KM indicates Kaplan-Meier.
