Abstract

A poly(amino acid)-based approach for scalable synthesis of micro-/mesoporous carbon (PC) with high specific surface area and narrow distribution of micro- and mesopores is presented. Using cross-linked poly aspartic acid as a precursor, PC was obtained by in situ one-step carbonization without the activating agent. The resulting PC had an ultrahigh adsorption capacity for H2 (4.43 wt %) and CH4 (4.49 mmol g–1). This novel method could significantly decrease the wastewater hazards caused by washout of the considerable amount of the activating agent. The PC showed promising application in gas adsorption and storage.
1. Introduction
Porous carbon (PC) is regarded as a promising gas storage material for energy-related applications with advantages of good chemical and thermal resistance, a diversity of texture and structure, abundance, and ease of processing.1 One of the obstacles to PC preparation is the complex and environmentally unfriendly method of synthesis.2 Generally, there are multiple steps performed in the conventional template process, which processed the design and synthesis of precursors, the combination of carbonization and activation, and molten salt synthesis routes.3 As a result, long carbonization and activation times are required during their preparation, causing the process to be energy-intensive.4 In addition, a considerable amount of activating agents such as alkali, chlorides, and acids is needed as oxidant and dehydrating agents to generate a large surface area and ordered microporosity in PCs suitable for physisorption of gas molecules through van der Waals forces.1,5 Nevertheless, after carbonization, the residues of the activating agents were then neutralized and washed using concentrated acid and a large amount of water. Thus, the preparation of PCs is environmentally unfriendly because the discharge of wastewater causes severe environmental consequences.
On the other hand, researchers have spared no effort in finding more sustainable, cost-effective approaches using biomaterials and biomass sources to make PC materials. Some methods utilized carbon- and nitrogen-rich precursors to simplify the carbonization stage.6,7 Except for the biomass resources, bacterial and other protein-rich compounds have been applied as precursors for PC production.8−14 Unfortunately, the composition of these precursors is complex and without defined chemical composition or clear arrangement of carbon atoms. This makes the surface pore size distribution uniformity of the carbonized product poor, thus making it difficult to improve its gas adsorption and electrical properties.
As described above, we selected polyaspartic acid (PASP) and its cross-linked product (CroPASP) as precursors for the preparation of PC. PASP is an amino acid-based polymer, with a long molecular chain and high nitrogen content. Because PASP has a strong hydrophilic character, one of the main application directions was preparing CroPASP for water-absorbing materials by cross-linking reaction.1,2,15 In previous work, PASP prepared from an environmentally friendly, low-cost method was applied in water treatment, agriculture, and medicine.16,17 Except for PASP, the CroPASP could also be prepared using “green” methods18 (Figure S1). When used as a precursor to PCs, PASP (PASP–K+) obtained using polysuccinimide (PSI) hydrolysis with KOH can provide a pore activation effect without use of an additional activator. Moreover, the K atom in PASP–K+ is more uniform in the material than the activator added by physical mixing. On the other hand, as a biopolymer material, PASP has a clear molecular structure and carbon chain arrangement, which makes its carbonization process regular and controllable. Therefore, the preparation process of carbonized-PASP (C-PASP) could be achieved in shorter carbonization and activation times, with less activator usage, and a larger specific surface area produced as a result;7,8,15 however, compared to PASP with most of its main chain in a random coiled state, CroPASP exhibits a more ordered and stable molecular structure because of the cross-linking between the main chains. Therefore, the pyrolysis products of CroPASP will have new surface characteristics, improved gas adsorption, and so forth. To our knowledge, PC derived from CroPASP has not been prepared and applied before. Therefore, in this study, we synthesized a carbonized CroPASP (C-CroPASP) with the CroPASP having been prepared by an environmentally friendly process for use as a precursor and without need for additional salt or alkali as activating agents and investigate its adsorption capacities for H2 and CH4.
2. Materials and Methods
2.1. Materials
Aspartic acid (ASP) was purchased from Changzhou Yabang Chemical Co., Ltd. Potassium hydroxide (KOH), phosphoric acid (H3PO4, 85 wt %), hydrochloric acid (HCl, 36 wt %), and silver nitrate (AgNO3) were obtained from Sinopharm Chemical Reagent Beijing Co., Ltd. Ethylene glycol diglycidyl ether (EGDGE, epoxy value ≥0.65) was purchased from Shanghai RuFa Chemical Technology Co., Ltd. Hollow carbon nanospheres were purchased from Aladdin, China. Mesoporous carbon was purchased from Sigma-Aldrich, Germany.
2.2. Preparation of PSI, PASP, and CroPASP
PSI was condensation polymerized by ASP using H3PO4 as a catalyst. According to previous work,19−22 the reaction was carried out at 210 °C under −0.09 MPa for 8 h, and the mass ratio of ASP to H3PO4 was 2:1. After the polymerized product was pulverized to 60–80 mesh, it was washed with deionized water until neutral and dried for 12 h at 120 °C. A specific PSI with a molecular weight (Mw) of 150,000 Da was applied (Mw/Mn = 2.31) (Figure S2 and Table S1).
PASP was prepared through hydrolysis of PSI in potassium hydroxide solution. Specifically, 6 g of PSI was added to 15 mL of deionized water containing 3.82 g of KOH (4.54 mol L–1, the molar ratio of PSI to potassium hydroxide was 1:1.1). Then, the solid–liquid mixture was stirred at 30 °C for 1 h. After this, the PASP solution was freeze-dried in vacuo at −54 °C for 36 h.
CroPASP was produced by PASP using EGDGE as the cross-linking agent.18 Briefly, the pH of the PASP solution was adjusted to 4.8 by hydrochloric acid, followed by mixing with 3 g of EGDGE (the molar ratio of PSI units to EGDGE was 3.6:1). The above mixture resulted in formation of a gel after stirring at 50 °C for 5 h. The CroPASP gel was freeze-dried in vacuo at −54 °C for 24 h. The synthesis process of the above three biopolymers is shown in Figure S1.
2.3. Synthesis of the PCs
PSI, PASP, and CroPASP were placed in a tube furnace under an N2 atmosphere. The temperature for precursor carbonization was set to 900 °C, and the duration thereat was 3 h. The heating rate was 5 °C min–1, and the nitrogen flow rate was 3.6 L h–1. After carbonization, the products were naturally cooled to below 30 °C, followed by washing with 1 M HCl (about 5–6 mL HCl per 1 g PCs) and distilled water until no chloride ions were detected with AgNO3. Then, the PSI, PASP, and CroPASP-derived PC specimens were dried in vacuo for 24 h at 80 °C and were called C-PSI, C-PASP, and C-CroPASP, respectively.
2.4. Characterization
A field-emission scanning electron microscope (SEM) (SU8000, Hitachi, Japan) and a transmission electron microscope (TEM) (H800, Hitachi, Japan) were used to analyze the microscopic features of the PCs. Thermogravimetric analysis was performed based on the STA449F3 apparatus (NETZSCH, Germany). Powder X-ray diffraction was carried out with an X-ray diffractometer (D8FOCUS, Bruker, Germany). Raman spectra were analyzed with a Raman Microscope (Renishaw, United Kingdom). X-ray photoelectron spectroscopy (XPS) spectra were obtained on an AXIS ULTRA spectrometer (Kratos Analytical, UK) using a monochromatized Al Kα X-ray source (1486.71 eV). Elemental analysis was conducted using a Vario EL cube (Elementar, Germany).
Surface areas and pore size distributions were measured by nitrogen adsorption and desorption at 77.3 K using an ASAP 2020 (Micromeritics) volumetric adsorption analyzer. The Brunauer–Emmette–Teller (BET) surface areas were calculated on the N2 adsorption branch at relative pressures (P/P0) ranging from 0.05 to 0.20. The surface area and volume of the micro/mesopores were obtained on the N2 adsorption branch using the t-plot method. The Harkins–Jura equations were used for calculation of the statistical thickness curves. The total pore volume was calculated with the amount adsorbed at a relative pressure P/P0 of around 0.99. The pore size distribution was determined based on the nonlocal density functional theory (NLDFT). Samples were degassed at 300 °C for 12 h in vacuo (10–5 bar) before analysis.
2.5. Gas Adsorption Measurements
Hydrogen and methane adsorption isotherms were measured using a static volumetric system (ASAP 2020, USA). A third-order virial expansion equation of the ideal gas law was employed in calculating the amount of gas adsorbed by the material, from its pressure, temperature, and the occupied volume. Before the adsorption measurements, approximately 100 mg of PC sample was activated in situ by heating at 300 °C for 12 h in vacuo (10–5 bar) to degas the sample. H2 adsorption measurements were carried out at liquid nitrogen temperature (77.3 K) and a pressure of up to 1.13 MPa. CH4 adsorption experiments were performed at 298 K and a pressure of up to 0.98 MPa.
3. Results and Discussion
3.1. Preparation of PCs
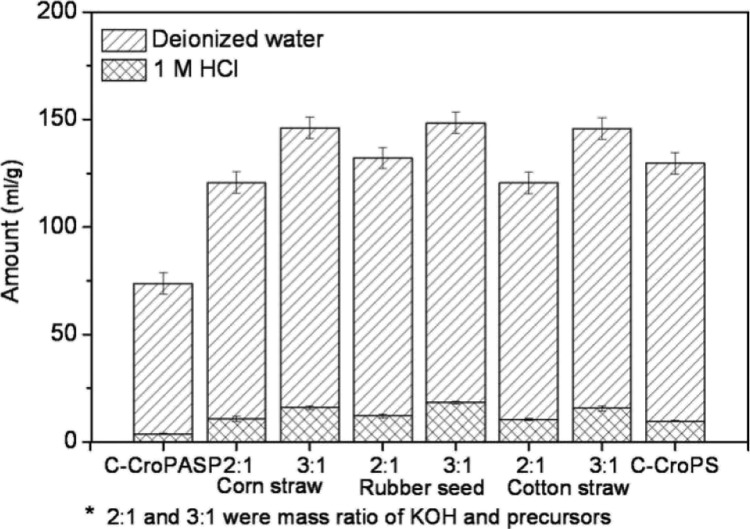
To overcome the aforementioned problems, we selected the cross-linked poly aspartic acid (CroPASP) hydrogel as the PC precursor for the first time (Figure 1). PASP is an amino acid-based polymer, with a long molecular chain and high nitrogen content. CroPASP is easy to prepare at a low cost and using environmentally friendly methods16−18 (the synthesis of CroPASP is shown in Figure S1). After cross-linking, the randomly twisted nonhydrophilic chains in the PSI molecule were changed into flexible hydrophilic chains with some secondary structures in the CroPASP structure, thus forming hydrophilic flexible networks (this conclusion comes from our hypothesis, and there is no experimental data for the time being). In the hydrolyzation process, carboxyl groups in PASP and the corresponding CroPASP were reacted with potassium hydroxide, so the potassium salts were more evenly distributed in the CroPASP structures in comparison with mechanically mixed conventional precursors (Figure S3 and Table S2). The functional K atom in CroPASP can affect the molecular chain and act as oxidant and dehydrating adjuvants. During carbonization at 900 °C, potassium hydroxide, the conventional activating agent, was replaced by the potassium salts in precursors, and therefore, the use of large amounts of activating agents is avoided. This also reduced the demand for HCl solution and deionized water, which was supposed to be used for PC washing after carbonization (Figure 2).
Figure 1.

Synthesis of PC by CroPASP.
Figure 2.
Amounts of HCl and deionized water required to produce PCs.
The straight PASP molecules and carbon chain arrangement are also regular and controllably carbonized. By cross-linking, CroPASP exhibits flexible hydrophilic chains with some secondary structures in comparison with the randomly twisted nonhydrophilic chains in PSI molecules, and therefore, no porogenic agent is required in preparing the novel CroPASP-derived PC that contains naturally formed meso/micropores.4,7,13
3.2. Morphology and Structural Characterization of PCs
The surface structure of C-CroPASP was further compared with the PCs from PSI and PASP precursors. SEM images indicated that the morphologies of the precursors and corresponding PCs were significantly different in each of the three groups (Figure S4). A smooth surface was obtained in the groups of PSI and PASP, which was caused by rigid molecular chains being rubbed together during solid-phase synthesis (Figure S4a). In contrast, the wrinkles and holes in PSI and PASP structures were caused by the entanglement and twisting of long molecular chains (Figure S4b). The spatial network of CroPASP showed many honeycomb structures (Figure S4c). As verified by thermogravimetric analysis (Figure S5), the PC features after carbonization depend on the structure of the precursor. Thus, the dissimilarity of PSI, PASP, and CroPASP template structures resulted in different morphologies after carbonization. As illustrated in Figure 3a,d, after carbonization, the C-PSI surface was smoother with fewer holes. It was attributed to the smooth and dense surface of PSI and the lack of activating agent. In addition, with no alkaline solution being used in PSI preparation, the activation of the PSI-derived PC was also deficient because of the limitations imposed by the activating agent. In contrast, PC structures with many pores were found in C-PASP and C-CroPASP after carbonization, which were attributed to the complex and fluffy structure of the precursors (Figure 3b,c). The potassium salts that participated in the carbonization process were conducive to the activation of the PCs, and because of the more fluffy and regular structure of CroPASP compared to that of PASP, more pores were also developed in C-CroPASP (Figure 3c). This was attributed to the exchange of matter and energy of the precursors facilitated during carbonization, thereby allowing more pores to have formed in C-CroPASP. TEM images also revealed that abundant micropores were created on the surface of C-PASP and C-CroPASP while few micropores occurred on the surface of C-PSI (Figure 3d–f).
Figure 3.
SEM images of C-PSI (a), C-PASP (b), and C-CroPASP (c); TEM images of C-PSI (d), C-PASP (e), and C-CroPASP (f).
XRD profiles showed two broad diffraction peaks at around 26 and 44° (Figure 4a), which could be assigned to (002) and (100) reflections of carbon materials, respectively.23 As can be seen from the figure, the (002) peak of C-PSI is relatively sharper than that of C-PASP and C-CroPASP, indicating a more ordered microstructure of C-PSI. After hydrolysis and cross-linking treatment, the structure gets more disordered (C-PASP and C-CroPASP). Raman spectroscopy was employed to inspect the structures of the PC samples. As shown in Figure 4b, the Raman spectra of PCs exhibit two broad peaks at 1348 and 1593 cm–1, which can be assigned to D (representing the disordered or defective feature) and G bands (the ordered feature) of the carbon materials, respectively, consistent with previous literature.24 The relative intensity of the ID/IG is usually used to evaluate the disordered degree of the carbon. The high value of 1.75 for C-CroPASP further indicates a more disordered microstructure, correlating well with the XRD analysis. In addition, the full-width at half maximum (fwhm) of D bands for the carbon specimens was 190, 250, and 273 cm–1. These results revealed that the degree of amorphous carbon was correlated with the precursor surface structure, thus in line with the XRD profiles.
Figure 4.
(a) XRD patterns; (b) Raman spectra; and (c) ID/IG and fwhm of the PCs.
XPS spectra showed that elemental C, O, and N were contained in the PC specimens (Figure 5a). The element contents were different in specimens of C-PSI from C-PASP and C-CroPASP (Figure 5b,c). The content of O was increased by ca. 50% after synthesizing the PASP; however, C and N contents showed no significant change. As expected, after carbonization, the elemental O content in C-PASP was also higher than that of C-PSI; however, as shown in Figure 5c, the elemental N content in C-CroPASP was higher than that in C-PASP, indicating that the reticulated carbon skeleton in the CroPASP structure showed beneficial effects in terms of N bonding after carbonization.
Figure 5.
XPS spectra of PCs. (a) XPS spectra for PCs; (b) high-resolution spectra of O 1s for PCs; (c) high-resolution spectra of N 1s for PCs; (d) high-resolution spectra of C 1s for C-PSI; (e) high-resolution spectra of C 1s for C-PASP; and (f) high-resolution spectra of C 1s for C-CroPASP.
The high-resolution XPS spectrum of C 1s for PC specimens was divided into four peaks (Figure 5d–f). As illustrated, the peak located at 284.7 eV was the characteristic of the sp2 carbon, and the other three peaks located at 285.4, 287.2, and 289.1 eV were assigned to the functional groups of C–O, C=O, and O–C=O, respectively.25 These results revealed that several groups such as hydroxyl and carboxyl might remain on the surface of PCs.
The surface functional groups and bonds of PCs were investigated using Fourier transform infrared (FT-IR) (Figure 6). The results in Figure 6 indicated that all of the PC specimens showed a group of peaks at 1420 to 1640 cm–1, which were assigned to the aromatic skeleton stretching mode.26 C=O stretching was hidden in the peak at 1640 cm–1. Moreover, a very broad peak appeared at 3500 cm–1, which was attributed to the O–H and N–H stretching vibration of carboxyl and amine groups.27 The presence of peaks at 1044, 1132, 1187, and 1217 cm–1, corresponding to the C–O stretching modes, confirmed the presence of several oxygenated functional groups on the surface of the PC specimens.28 The peaks between 560 and 700 cm–1 were the response of the CH peak in the aromatic ring.29 The peaks shown in the FT-IR spectra generally coincided with the XPS results. In addition, the intensity changes of each peak in FT-IR spectra also confirmed the change in proportion of amorphous carbon (in the ascending order) as: C-PSI, C-PASP, and then C-CroPASP.
Figure 6.

FT-IR spectra of the PCs.
3.3. Porosities of PCs
The porous properties of PCs were investigated by nitrogen adsorption at 77.3 K. As shown in Figure 7a, because of the smooth surface and fewer holes in C-PSI, the N2 adsorption of C-PSI is quite small (10.37 cm3 g–1). The N2 adsorption curve of C-PASP was a type I adsorption isotherm curve, thus equivalent to the monolayer reversible Langmuir adsorption isotherm.30 Besides, the curve evinced a rapid uptake of N2 at low relative pressures (P/P0 < 0.001), indicating that substantial micropores were preserved in C-PSI. In contrast, a steep rise was obtained in C-PASP at high relative pressures (P/P0 > 0.9), indicating the presence of mesopores or macropores in PC;31 however, the adsorption isotherm of C-CroPASP differed from that of other PC specimens. As can been seen from Figure 7a, a hysteresis loop was evident in the C-CroPASP curve. According to the adsorption/desorption curve and shape of this hysteresis loop, this curve was a type IV curve, and the hysteresis loop was akin to that of an H2 type.30 The hysteresis loop arose over a range of high values of P/P0. In mesopores, capillary condensation was also observed. The curve of C-CroPASP displays a rapid uptake at low relative pressures. This phenomenon is attributed to multilayer adsorption.32 Combining with the SEM and TEM results, it was suggested that C-CroPASP had a complex but regular multilayer hole structure. This unique structure illustrates that the cross-linking reaction contributed to the changing of the polymer chain into a spatial network from random distortion. The carbon skeletons (after carbonization) were arranged in a more orderly manner, indicating that the C-CroPASP had a better gas adsorption capacity.33
Figure 7.
(a) N2 adsorption/desorption isotherms and (b) pore size distribution curves of PCs.
The pore size distribution curves of the PCs were calculated based on NLDFT. As shown in Figure 7b, there were almost no holes in C-PSI, except for a few pores with a diameter of around 0.57 nm. C-PASP contains many micropores with a pore diameter of around 0.52 nm, indicating that C-PASP had a type I gas adsorption isotherm. It was interesting to note that the pore size distribution of C-CroPASP was complex; many micropores with a diameter of around 0.60 nm and some mesopores with a diameter of around 3.97 nm were found. The presence of the two aforementioned types of pores made the surface of C-CroPASP rich in multilayer composite pores, which resulted in the hysteresis loop in the gas adsorption isotherm. Both BET surface area and total pore volume were increased in the following order: C-PSI, C-PASP, and then C-CroPASP (Table 1). The activating agent in PASP was in the form of −COOK (Figure S1), so the surface of C-PASP contained a large number of micropores, whereas mesopores occurred from the release of CO2 by KOH via K2CO3.34−37 Besides, the larger surface area and pore volume of C-CroPASP were attributed to the synergistic effect of −COOK as the activating group and the regular spatial network structure of the molecular chains in CroPASP. Pore properties and elemental contents of PCs are listed in Table 1 (the co-existence of graphitic and disordered morphologies was also confirmed by XRD and Raman spectroscopy, as shown in Figure 4). The surface functional groups were analyzed using XPS and FT-IR (Figures 5 and 6, respectively).
Table 1. Pore Properties and Content of Elements of the PCs.
| content
of elements [wt %]e |
|||||||
|---|---|---|---|---|---|---|---|
| Samples | SBETa (m2 g–1) | Smicrob (m2 g–1) | VTotalc (cm3 g–1) | Vmicrod (cm3 g–1) | C | O | N |
| C-PSI | 6.49 ± 0.13 | 5.38 ± 0.16 | 0.016 ± 0.004 | 0.0019 ± 0.0010 | 87.96 | 8.64 | 3.40 |
| C-PASP | 952 ± 19 | 787 ± 16 | 0.61 ± 0.03 | 0.42 ± 0.03 | 87.23 | 11.33 | 1.44 |
| C-CroPASP | 1458 ± 17 | 1200 ± 20 | 1.13 ± 0.04 | 0.64 ± 0.04 | 90.52 | 7.74 | 1.75 |
BET surface area.
Micropore surface area.
Total pore volume.
Micropore volume.
Data obtained by XPS.
3.4. Gas Adsorption Performance of PCs
We further investigated the hydrogen and methane adsorption properties of PCs at 77.3 K and 1.13 bar. As shown in Figure 8, both C-PASP and C-CroPASP exhibited a higher H2 and CH4 adsorption capacity than C-PSI because of the smaller surface area and less porous structure in the C-PSI. Some 4.43 wt % of H2 could be absorbed by C-CroPASP (Figure 8a) as was mainly attributed to the narrow distribution of micropores on the surface of C-CroPASP, making it suitable for the binding and adsorption of smaller gas molecules such as H21. Moreover, the complex hierarchical pore structure (0.60 and 3.97 nm) could further improve the performance of the gas adsorption of C-CroPASP.38 Compared with other hydrogen absorbing materials, C-CroPASP generally absorbs more hydrogen than metal hydrides but lower than MOF.39−41 Although C-CroPASP has lower hydrogen absorption than MOF, its cost and stability are better than MOF.42,43 This excellent hydrogen storage performance was also reflected in comparison with other PCs. Compared with the H2 adsorption performance of commercialized PCs such as hollow carbon nanospheres (Aladdin, China) and mesoporous carbon (Sigma-Aldrich, German) under similar conditions, C-CroPASP showed an ultrahigh rate of H2 absorption compared with other research5,44−49 (Figure 9 and Table S3). The dashed green line (Figure 9) is mainly used to distinguish the hydrogen absorption of PC prepared with CroPASP as the precursor and the hydrogen absorption of conventional PC.
Figure 8.
(a) H2 sorption curves collected (77.3 K up to 1.13 bar); (b) CH4 sorption curves collected (273 K up to 0.98 bar).
Figure 9.

Current advances in H2 adsorption capacity at 77.3 K up to 1.13 bar.
The methane sorption performance of the samples was also explored. Based on the similarity in their hydrogen uptakes, these PCs also showed good methane adsorption capacities, of which C-PASP reached 2.46 mmol g–1, and C-CroPASP reached 4.49 mmol g–1, exceeding or equivalent to the traditional material under the same conditions (Figure 8b). These results exceed or correspond to most reported PC materials under the same conditions, such as the nitrogen-doped PC of CHCPB-K-700 (2.76 mmol g–1, 273 K, 1.13 bar),5 graphene oxide/ordered mesoporous carbon (2.1 mmol g–1, 293 K, 1.01 bar),50 porous sulphur- and oxygen-codoped carbon (1.69 mmol g–1, 273 K, 1.0 bar),51 and nitrogen-doped carbon nanoribbons (1.875 mmol g–1, 273 K, 1.0 bar)52 (Table S4).
The novel activating agent-free method for C-CroPASP is also an in situ, one-step carbonization process, which offers significant advantages. First, potassium salts were evenly distributed in the template structure as an activating agent. In contrast, in a conventional carbonization process, potassium hydroxide was physically blended with the hydrochar in the solid phase. Hence, the activation of the current one-step synthesis was more effective because the potassium salts showed deeper binding with the template structure rather than with the surface of the char granulum in the conventional process. Second, the demand for potassium hydroxide in the current carbonization process was negligible. In a conventional activation process, the potassium hydroxide dosage rate was always higher than the mass of char template to ensure modification of the pore size of the char.53 In contrast, the etching effect of K was improved because of the even distribution of K in the template structure. Thus, no additional activating agent was required. Third, without using an additional activating agent, the demand for large amounts of water and acid used when washing PCs was obviated, thus making the in situ, one-step carbonization process more environmentally friendly. Fourth, the carbonization and activation steps were combined, therefore the energy requirement for PC production was reduced (complex temperature readjustment was also avoided). Finally, the cost of the CroPASP template, which is currently abundantly applied in wastewater treatment and agricultural applications, is much cheaper than other synthetic materials.
4. Conclusions
C-CroPASP was prepared based on a novel activating agent-free method. This method could not only improve the H2 (4.43 wt %) and CH4 (4.49 mmol g–1) adsorption properties of the PC products but also avoided the use of large amounts of activating agent and waste water discharge. As expected, the CroPASP hydrogel obtained by PASP cross-linking in water had a spatial network structure. A secondary pore structure on the surface (0.60 and 3.97 nm) and uniform distribution of pore size were obtained, which also provided excellent H2 and CH4 adsorption properties. The current work opened new possibilities for environmentally friendly PC production with promising applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21865026); Xinjiang Production and Construction Corps Project (2018BC005); Opening Project of State Key Laboratory of Organic–Inorganic Composites (oic-201901008); Hebei Key Research and Development Fund project (18273401D); and Hebei Province’s Innovation Capacity Improvement Project (18952814D).
Glossary
Abbreviations
- PC
porous carbon
- PSI
polysuccinimide
- PASP
poly aspartic acid
- CroPASP
cross-linked poly aspartic acid
- C-PSI
porous carbon by PSI
- C-PASP
porous carbon by PASP
- C-CroPASP
porous carbon by CroPASP
- PC-Alddin
porous carbon by hollow carbon nanospheres (Aladdin, China)
- PC-SigmaAldrich
porous carbon by mesoporous carbon (Sigma-Aldrich, Germany)
- Da
Dalton
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04110.
Flowchart for CroPASP preparation from PSI; GFC chromatogram of PSI; result of PSI molecular weight; EDS histographs and mapping of CroPASP; elemental composition of CroPASP studied by EDS analysis; SEM images of PSI, PASP, and CroPASP; TG and DTG of PSI, PASP, and CroPASP; H2 adsorption capacity of C-PASP and C-CroPASP compared with published values; and CH4 adsorption capacity of C-PASP and C-CroPASP compared with published values (PDF)
Author Contributions
The manuscript was written through contributions of all the authors. All the authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sevilla M.; Mokaya R. Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. 10.1039/c3ee43525c. [DOI] [Google Scholar]
- Zhou X.; Liao Q.; Bai T.; Yang J. Nitrogen-doped microporous carbon from polyaspartic acid bonding separator for high performance lithium-sulfur batteries. J. Electroanal. Chem. 2017, 791, 167–174. 10.1016/j.jelechem.2017.03.004. [DOI] [Google Scholar]
- Zou K.; Deng Y.; Chen J.; Qian Y.; Yang Y.; Li Y.; Chen G. Hierarchically porous nitrogen-doped carbon derived from the activation of agriculture waste by potassium hydroxide and urea for high-performance supercapacitors. J. Power Sources 2018, 378, 579–588. 10.1016/j.jpowsour.2017.12.081. [DOI] [Google Scholar]
- Li B.; Cheng Y.; Dong L.; Wang Y.; Chen J.; Huang C.; Wei D.; Feng Y.; Jia D.; Zhou Y. Nitrogen doped and hierarchically porous carbons derived from chitosan hydrogel via rapid microwave carbonization for high-performance supercapacitors. Carbon 2017, 122, 592–603. 10.1016/j.carbon.2017.07.009. [DOI] [Google Scholar]
- Zhang C.; Kong R.; Wang X.; Xu Y.; Wang F.; Ren W.; Wang Y.; Su F.; Jiang J.-X. Porous carbons derived from hypercrosslinked porous polymers for gas adsorption and energy storage. Carbon 2017, 114, 608–618. 10.1016/j.carbon.2016.12.064. [DOI] [Google Scholar]
- Jeon J.-W.; Sharma R.; Meduri P.; Arey B. W.; Schaef H. T.; Lutkenhaus J. L.; Lemmon J. P.; Thallapally P. K.; Nandasiri M. I.; McGrail B. P.; Nune S. K. In situ one-step synthesis of hierarchical nitrogen-doped porous carbon for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7214–7222. 10.1021/am500339x. [DOI] [PubMed] [Google Scholar]
- Ma G.; Wu Y.; Sun K.; Peng H.; Wang H.; Lei Z. High performance nitrogen-doped carbon for supercapacitor obtained by carbonizing eco-friendly and cheap polyaspartic acid. Mater. Lett. 2014, 132, 41–44. 10.1016/j.matlet.2014.06.028. [DOI] [Google Scholar]
- Ramakrishnan P.; Shanmugam S. Nitrogen-doped carbon nanofoam derived from amino acid chelate complex for supercapacitor applications. J. Power Sources 2016, 316, 60–71. 10.1016/j.jpowsour.2016.03.061. [DOI] [Google Scholar]
- Rana M.; Subramani K.; Sathish M.; Gautam U. K. Soya derived heteroatom doped carbon as a promising platform for oxygen reduction, supercapacitor and CO2 capture. Carbon 2017, 114, 679–689. 10.1016/j.carbon.2016.12.059. [DOI] [Google Scholar]
- Liu Y.; Huang B.; Lin X.; Xie Z. Biomass-derived hierarchical porous carbons: boosting the energy density of supercapacitors via an ionothermal approach. J. Mater. Chem. A 2017, 5, 13009–13018. 10.1039/c7ta03639f. [DOI] [Google Scholar]
- Jeong H.; Kim H. J.; Lee Y. J.; Hwang J. Y.; Park O.-K.; Wee J.-H.; Yang C.-M.; Ku B.-C.; Lee J. K. Amino acids derived nitrogen-doped carbon materials for electrochemical capacitive energy storage. Mater. Lett. 2015, 145, 273–278. 10.1016/j.matlet.2015.01.067. [DOI] [Google Scholar]
- Sun D.; Ban R.; Zhang P.-H.; Wu G.-H.; Zhang J.-R.; Zhu J.-J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. 10.1016/j.carbon.2013.07.095. [DOI] [Google Scholar]
- Hou J.; Cao C.; Idrees F.; Ma X. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. 10.1021/nn506394r. [DOI] [PubMed] [Google Scholar]
- Xia Y.; Fang R.; Xiao Z.; Huang H.; Gan Y.; Yan R.; Lu X.; Liang C.; Zhang J.; Tao X.; Zhang W. Confining sulfur in N-doped porous carbon microspheres derived from microalgaes for advanced lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 23782–23791. 10.1021/acsami.7b05798. [DOI] [PubMed] [Google Scholar]
- Sun K.; Feng E.; Peng H.; Ma G.; Wu Y.; Wang H.; Lei Z. A simple and high-performance supercapacitor based on nitrogen-doped porous carbon in redox-mediated sodium molybdate electrolyte. Electrochim. Acta 2015, 158, 361–367. 10.1016/j.electacta.2015.01.185. [DOI] [Google Scholar]
- Yang J.; Wang F.; Fang L.; Tan T. Synthesis, characterization and application of a novel chemical sand-fixing agent-poly(aspartic acid) and its composites. Environ. Pollut. 2007, 149, 125–130. 10.1016/j.envpol.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Wei J.; Yang H.; Cao H.; Tan T. Using polyaspartic acid hydro-gel as water retaining agent and its effect on plants under drought stress. Saudi J. Biol. Sci. 2016, 23, 654–659. 10.1016/j.sjbs.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H.; Zhang X.; Chen Q.; Wei J.; Wang Y.; Dong A.; Yang H.; Tan T.; Cao H. Preparation of poly(aspartic acid) superabsorbent hydrogels by solvent-free processes. J. Polym. Eng. 2015, 35, 647–655. 10.1515/polyeng-2014-0275. [DOI] [Google Scholar]
- Wang Y.; Xue M.; Wei J.; Li C.; Zhang R.; Cao H.; Yang J.; Tan T. Novel solvent-free synthesis and modification of polyaspartic acid hydrogel. RSC Adv. 2012, 2, 11592–11600. 10.1039/c2ra20661g. [DOI] [Google Scholar]
- Zhao Y.; Su H.; Fang L.; Tan T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. 10.1016/j.polymer.2005.04.015. [DOI] [Google Scholar]
- Cao H.; Ma X.; Sun S.; Su H.; Tan T. A new photocrosslinkable hydrogel based on a derivative of polyaspartic acid for the controlled release of ketoprofen. Polym. Bull. 2010, 64, 623–632. 10.1007/s00289-009-0215-z. [DOI] [Google Scholar]
- Cheng H.; Li Y.-Y.; Zeng X.; Sun Y.-X.; Zhang X.-Z.; Zhuo R.-X. Protamine sulfate/poly(l-aspartic acid) polyionic complexes self-assembled via electrostatic attractions for combined delivery of drug and gene. Biomaterials 2009, 30, 1246–1253. 10.1016/j.biomaterials.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Srinivas G.; Burress J.; Yildirim T. Graphene oxide derived carbons (GODCs): synthesis and gas adsorption properties. Energy Environ. Sci. 2012, 5, 6453–6459. 10.1039/c2ee21100a. [DOI] [Google Scholar]
- Yang X.; Yu M.; Zhao Y.; Zhang C.; Wang X.; Jiang J.-X. Remarkable gas adsorption by carbonized nitrogen-rich hypercrosslinked porous organic polymers. J. Mater. Chem. A 2014, 2, 15139–15145. 10.1039/c4ta02782e. [DOI] [Google Scholar]
- Wang H.; Sun X.; Liu Z.; Lei Z. Creation of nanopores on graphene planes with MgO template for preparing high-performance supercapacitor electrodes. Nanoscale 2014, 6, 6577–6584. 10.1039/c4nr00538d. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Yang Q.; Yang X.; Deng Y. One-step synthesis of in-situ N-doped ordered mesoporous titania for enhanced gas sensing performance. Microporous Mesoporous Mater. 2018, 270, 75–81. 10.1016/j.micromeso.2018.04.008. [DOI] [Google Scholar]
- Shahraki S.; Samareh Delarami H. Magnetic chitosan-(d-glucosimine methyl)benzaldehyde Schiff base for Pb+2 ion removal. Experimental and theoretical methods. Carbohydr. Polym. 2018, 200, 211–220. 10.1016/j.carbpol.2018.07.081. [DOI] [PubMed] [Google Scholar]
- Weinberger C.; Cao X.; Tiemann M. Selective surface modification in bimodal mesoporous CMK-5 carbon. J. Mater. Chem. A 2016, 4, 18426–18431. 10.1039/c6ta07772b. [DOI] [Google Scholar]
- Wang F.; Ren F.; Mu P.; Zhu Z.; Sun H.; Ma C.; Xiao C.; Liang W.; Chen L.; Li A. Hierarchical porous spherical-shaped conjugated microporous polymers for the efficient removal of antibiotics from water. J. Mater. Chem. A 2017, 5, 11348–11356. 10.1039/c7ta02982a. [DOI] [Google Scholar]
- Sing K. S. W.; Everett D. H.; Haul R. A. W.; Moscou L.; Pierotti R. A.; Rouquerol J.; Siemieniewska T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Jiang J.-X.; Su F.; Trewin A.; Wood C. D.; Niu H.; Jones J. T. A.; Khimyak Y. Z.; Cooper A. I. Synthetic control of the pore dimension and surface area in conjugated microporous polymer and copolymer networks. J. Am. Chem. Soc. 2008, 130, 7710–7720. 10.1021/ja8010176. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Jiang H.; Wang Q.; Zheng J.; Meng C. Kelp-derived three-dimensional hierarchical porous N,O-doped carbon for flexible solid-state symmetrical supercapacitors with excellent performance. Appl. Surf. Sci. 2018, 447, 876–885. 10.1016/j.apsusc.2018.04.061. [DOI] [Google Scholar]
- Shao L.; Liu M.; Huang J.; Liu Y.-N. CO2 capture by nitrogen-doped porous carbons derived from nitrogen-containing hyper-cross-linked polymers. J. Colloid Interface Sci. 2018, 513, 304–313. 10.1016/j.jcis.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Wang J.; Kaskel S.; Kaskel S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. 10.1039/c2jm34066f. [DOI] [Google Scholar]
- Otowa T.; Tanibata R.; Itoh M. Production and adsorption characteristics of MAXSORB: high-surface-area active carbon. Gas Sep. Purif. 1993, 7, 241–245. 10.1016/0950-4214(93)80024-q. [DOI] [Google Scholar]
- Lozano-Castelló D.; Calo J. M.; Cazorla-Amorós D.; Linares-Solano A. Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon 2007, 45, 2529–2536. 10.1016/j.carbon.2007.08.021. [DOI] [Google Scholar]
- Raymundo-Piñero E.; Azaïs P.; Cacciaguerra T.; Cazorla-Amorós D.; Linares-Solano A.; Béguin F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organization. Carbon 2005, 43, 786–795. 10.1016/j.carbon.2004.11.005. [DOI] [Google Scholar]
- Kobielska P. A.; Telford R.; Rowlandson J.; Tian M.; Shahin Z.; Demessence A.; Ting V. P.; Nayak S. Polynuclear complexes as precursor templates for hierarchical microporous graphitic carbon: an unusual approach. ACS Appl. Mater. Interfaces 2018, 10, 25967–25971. 10.1021/acsami.8b10149. [DOI] [PubMed] [Google Scholar]
- Darzi A. A. R.; Afrouzi H. H.; Moshfegh A.; Farhadi M. Absorption and desorption of hydrogen in long metal hydride tank equipped with phase change material jacket. Int. J. Hydrogen Energy 2016, 41, 9595–9610. 10.1016/j.ijhydene.2016.04.051. [DOI] [Google Scholar]
- Kwak Y. J.; Park H. R.; Song M. Y. Changes in microstructure, phases, and hydrogen storage characteristics of metal hydro-borate and nickel-added magnesium hydride with hydrogen absorption and release reactions. Int. J. Hydrogen Energy 2017, 42, 1018–1026. 10.1016/j.ijhydene.2016.10.097. [DOI] [Google Scholar]
- Nanba Y.; Tsutsumi T.; Ishimoto T.; Koyama M. Theoretical study of the hydrogen absorption mechanism into a palladium nanocube coated with a metal-organic framework. J. Phys. Chem. C 2017, 121, 14611–14617. 10.1021/acs.jpcc.7b03137. [DOI] [Google Scholar]
- Dhakshinamoorthy A.; Asiri A. M.; García H. Metal-organic framework (MOF) compounds: photocatalysts for redox reactions and solar fuel production. Angew. Chem., Int. Ed. 2016, 55, 5414–5445. 10.1002/anie.201505581. [DOI] [PubMed] [Google Scholar]
- Li C.; Qiao Y.; Lin X.; Che G. Preparation of quantum dots@ metal-organic frameworks and its application in the field of photocatalytic degradation. Prog. Chem. 2018, 30, 1308–1316. [Google Scholar]
- Sethia G.; Sayari A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. 10.1016/j.carbon.2015.12.032. [DOI] [Google Scholar]
- Yang S. J.; Kim T.; Im J. H.; Kim Y. S.; Lee K.; Jung H.; Park C. R. MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity. Chem. Mater. 2012, 24, 464–470. 10.1021/cm202554j. [DOI] [Google Scholar]
- Kim H. S.; Kang M. S.; Yoo W. C. Highly enhanced gas sorption capacities of N-doped porous carbon spheres by hot NH3 and CO2 treatments. J. Phys. Chem. C 2015, 119, 28512–28522. 10.1021/acs.jpcc.5b10552. [DOI] [Google Scholar]
- Zhang C.; Geng Z.; Cai M.; Zhang J.; Liu X.; Xin H.; Ma J. Microstructure regulation of super activated carbon from biomass source corncob with enhanced hydrogen uptake. Int. J. Hydrogen Energy 2013, 38, 9243–9250. 10.1016/j.ijhydene.2013.04.163. [DOI] [Google Scholar]
- Wróbel-Iwaniec I.; Díez N.; Gryglewicz G. Chitosan-based highly activated carbons for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 5788–5796. 10.1016/j.ijhydene.2015.03.034. [DOI] [Google Scholar]
- Liu X.; Zhang C.; Geng Z.; Cai M. High-pressure hydrogen storage and optimizing fabrication of corncob-derived activated carbon. Microporous Mesoporous Mater. 2014, 194, 60–65. 10.1016/j.micromeso.2014.04.005. [DOI] [Google Scholar]
- Szczęśniak B.; Choma J.; Jaroniec M. Effect of graphene oxide on the adsorption properties of ordered mesoporous carbons toward H2, C6H6, CH4 and CO2. Microporous Mesoporous Mater. 2018, 261, 105–110. 10.1016/j.micromeso.2017.10.054. [DOI] [Google Scholar]
- Zhang P.-Y.; Sui Z.-Y.; Wang E.-J.; Liu Y.-W.; Han B.-H. Preparation of hierarchically porous sulfur- and oxygen-co-doped carbon for gas uptake and lithium-ion battery. Microporous Mesoporous Mater. 2018, 264, 118–124. 10.1016/j.micromeso.2018.01.007. [DOI] [Google Scholar]
- Yang Q.-S.; Sui Z.-Y.; Liu Y.-W.; Han B.-H. Porous nitrogen-doped carbon nanoribbons for high-performance gas adsorbents and lithium ion batteries. Ind. Eng. Chem. Res. 2016, 55, 6384–6390. 10.1021/acs.iecr.6b00680. [DOI] [Google Scholar]
- Wang J.; Senkovska I.; Kaskel S.; Liu Q. Chemically activated fungi-based porous carbons for hydrogen storage. Carbon 2014, 75, 372–380. 10.1016/j.carbon.2014.04.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.