Figure 1. Major pathways of NO synthesis, metabolism, and clearance.
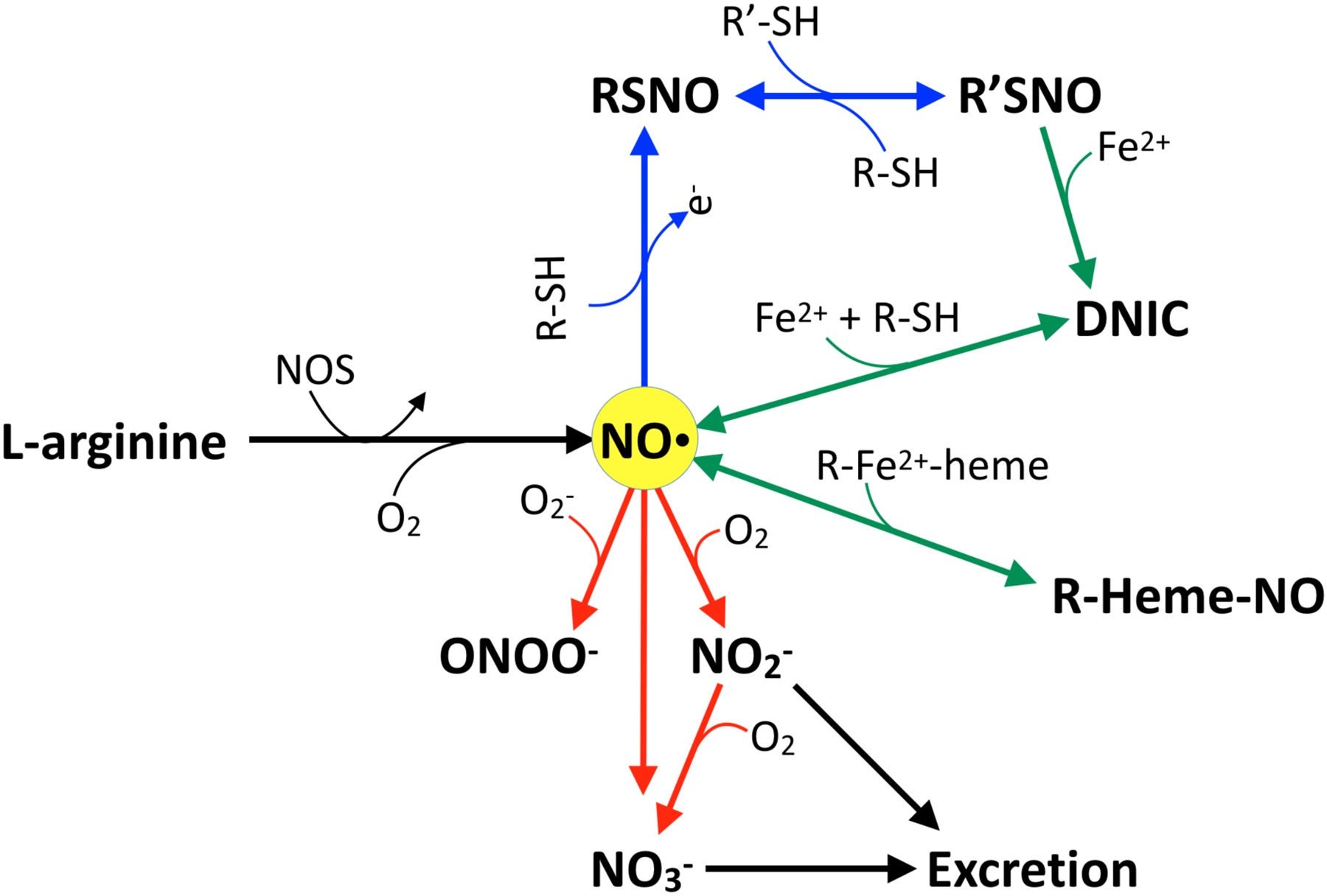
De novo NO is produced from L-arginine by NO synthases (NOS), a reaction that requires O2. If oxidized, NO free radical (NO·) can form nitrosothiols (RSNO), which are capable of transferring the NO moiety from one thiol to another (blue arrows). NO· and nitrosothiols can also react with heme and nonheme iron to produce heme iron nitrosyl compounds (R-heme-NO) and dinitrosyl iron complexes (DNIC) (green arrows). Finally, NO· can react with superoxide (O2−) to produce peroxynitrite (ONOO−), or with O2 to produce nitrite (NO2−) or nitrate (NO3−), both of which are excreted in the urine (red arrows). Note that NO3− can also be reduced back into NO2− by oral bacterial reductases, and that NO2− can be reduced back into NO under hypoxic and acidic conditions as described in the text.
