ABSTRACT
Background: The incidence of TB among Inuit is the highest in Canada. A significantly shorter latent TB infection (LTBI) treatment with rifapentine and isoniazid once weekly for 12 weeks (3HP) is now available in limited settings in Canada.
Methods: A prospective open-label 2-year observational postmarketing study was conducted introducing 3HP for the first time in Canada in Iqaluit followed by a program rollout in Qikiqtarjuaq, Nunavut.
Results: A total of 247 people were offered 3HP, 102 in the Iqaluit postmarketing study and 145 in the Qikiqtarjuaq program roll out. Although statistical significance was not reached, more people who started treatment completed treatment in the 3HP group (Iqaluit, 60/73 (82.2%) and Qikiqtarjuaq, 89/115 (77.4%)) than in the historical control 9INHgroup (306/420 = 72.9%) (p = 0.2). Most of the adverse events in 3HP treated patients were associated with mild discomfort but no disruption of normal daily activity. Not drinking alcohol was associated with increased 3HP completion (OR 13.33, 95% CI, 2.27–78.20) as was not taking concomitant medications (OR 7.19, 95% CI, 1.47–35.30).
Conclusions: The present study supports the feasibility and safety profile of 3HP for the treatment of LTBI in Nunavut.
KEYWORDS: Inuit, tuberculosis, latent tuberculosis infection treatment
Introduction
In 2017, the tuberculosis (TB) incidence among Inuit in Canada was approximately 300 times higher than the Canadian born non Indigenous population (170 vs 0.5 cases per 100,000) [1]. On World TB day, 24 March 2018, Inuit Tapiriit Kanatami (ITK) and the Government of Canada committed to reducing active TB cases by at least 50% by 2025 and to the elimination of TB in the Inuit Nunangat (Inuit homeland in Canada) by 2030 [2]. In order to achieve this laudable target, prevention of active TB disease will be paramount. Treatment of latent TB infection (LTBI) is one of the most important approaches we currently have to prevent active TB disease in order to reach TB elimination [3]. LTBI screening and treatment have been associated with decreases in TB among Indigenous populations in North America [4].
Current barriers to LTBI treatments are the length (number of doses) and side effects [5]. Due to some of these barriers and others, a recent meta-analysis of studies that reported on the latent TB cascade of care demonstrated that of those who start LTBI treatment 62% complete treatment [6]. The remote arctic communities of the Inuit Nunangat face additional challenges including language and cultural differences as well as significant disparities of the social determinants of health compared to the rest of the country [7]. Remote arctic settings have a chronic lack of human resources to administer treatments which results in high burnout among health-care workers, high turnover of staff and high cost of delivering care in these regions of Canada [8].
The current international standard for the treatment of LTBI is 6–9 months of Isoniazid (INH) [9]. Adherence to this lengthy regimen is one of the greatest impediments to treatment. A landmark multi-centred, multi-national randomised non-inferiority trial with approximately 4,000 adult patients per arm demonstrated that rifapentine and INH (3HP) given once weekly for a total of 12 doses was as effective as 9 months (252 doses) of daily INH treatment for LTBI [10]. These findings were also replicated in a large paediatric cohort nested within the larger trial [11]. The efficacy and safety of this new regimen have been established with phase 3 [10–12], phase 4 clinical trials [13,14] and observational studies [14–18] in addition to a network meta-analysis on efficacy and completion rates [19] and a systematic review of rifapentine adverse events compared to other treatments [20] done by our group.
While 3HP is becoming a standard of care in many countries including the United States [21], this regimen is still only available in Canada under restricted conditions [22]. Given the significant operational challenges encountered in this region of Canada we conducted a postmarketing study in Iqaluit, Nunavut followed by a program roll out in a second community, Qikiqtarjuaq, compared to historical controls from Iqaluit treated with 9 months of twice weekly INH (9INH) [23], to determine the feasibility of 3HP based on treatment completion and the safety profile of the regimen.
Methods
Setting
In 2016, the population of the Territory of Nunavut in the Canadian Arctic was estimated at 37,082, with Inuit representing 84% of the total population [24]. Iqaluit is the capital and largest community in Nunavut with a population of 7,590 people and 55% of the population of Iqaluit are Inuit, whereas Qikiqtarjuaq has a population of 616 people of which 93% are Inuit [24]. Iqaluit and Qikiqtarjuaq can only be accessed by plane or ship during the brief summer and by plane only during the winter months.
Patients and study type
Consecutive males or non-pregnant, non-nursing females between the ages of 2–65 years who were residents of Iqaluit, Nunavut who were diagnosed with LTBI between June 2016 – June 2018 or who had been previously diagnosed with LTBI that was not treated were invited to participate in the postmarketing study in Iqaluit. Patients were given the choice of [1]: the standard LTBI regimen in Nunavut, twice weekly isoniazid for 9 months (9INH) plus 25 mg of vitamin B6 directly observed preventive therapy (DOPT) or [2] once a week 3HP given DOPT for 12 weeks with a maximum dose of rifapentine 900 mg plus INH to a maximum of 900 mg plus 25 mg of vitamin B6 or [3] no treatment (education provided on seeking care if signs/symptoms of active TB disease were to develop). All treatment was provided free of charge. Directly observed therapy was done by registered nurses and licenced practical nurses primarily at the clinic and only if needed at the home to ensure that each dose of the medication was taken. At the time of starting the study, Health Canada had not approved rifapentine therefore Health Canada approval (no objection letter) was obtained to proceed with the study.
The original design of the Iqaluit study was an interrupted time series [25] however during the course of the study, the Government of Canada made rifapentine available by listing it on the list of drugs for urgent public health need [15]. The Government of Nunavut obtained access to rifapentine through this mechanism which then prompted the decision to stop the Iqaluit study before the sample size had been attained. The Department of Health from the Government of Nunavut decided to roll out 3HP through regular practice in a community-wide screening program in Qikiqtarjuaq between February and August 2018.
(See supplement for detailed inclusion/exclusion criteria and baseline testing)
Outcome measurements
The primary outcome was feasibility which was measured by the proportion of patients who completed 3HP treatment compared to the standard of 9 months INH of twice weekly prior to the start of the 3HP program [23]. The term “offered” was defined as when a TB physician offered treatment after taking a history, physical exam and baseline blood work. The term “started” was defined as taking at least one dose of 3HP. The term “completion” was defined as a minimum of 11 doses out of 12 in 16 weeks (90% completion) as per published RCTs [10]. In order to compare completion rates between 3HP and 9INH we used 90% completion of INH defined as ≥70/78 doses of the INH twice weekly given DOPT over 12 months. The safety profile of the regimen was measured using detailed records of any possibly related or related adverse events. The grading of the severity of the adverse events used a five-point scale (supplement).
Analysis
Patient demographics, treatment indication, medical conditions and risk factors were described. Treatment start and completion data were collected among all patients starting INH for LTBI in Iqaluit between January 2010-March 2016 and compared to the prospective data collected using the 3HP regimen. The proportion of participants who were offered and started and those that started and completed the 3HP regimen from Iqaluit and Qikiqtarjuaq were compared to the previous 6 years of 9INH treatment in Iqaluit using a chi-squared test significance set at a level of p ≤ 0.05. Univariable and multivariable logistic regression models were used to calculate the odds ratio to determine factors associated with the completion of 3HP treatment among patients starting treatment. The Firth correction was applied to the multivariate model. We included relevant demographic, clinical and risk factors that might affect the ability of the participant to complete treatment. Analysis was done using SAS V.9 by SAS Institute, Cary, North Carolina, USA.
The Iqaluit study was approved by the Ottawa Health Sciences Network Research Ethics Board. A research licence was obtained from the Nunavut Research Institute. Written informed consent was obtained from all participants in Iqaluit. In the roll out in Qikiqtarjuaq, verbal consent was obtained in accordance with the Department of Health policy.
Results
Among participants in Iqaluit who started 3HP (n = 73), mean age was 31 years, 69% were of Inuit ethnicity, none tested positive for HIV, hepatitis B or C (Table 1). All had normal chest radiographs and 92% were able to produce sputum. None of the participants had other risk factors for the development of active TB including being underweight, transplant, silicosis, or cancer. Only two participants had diabetes. Half of the participants who started 3HP were on other medications. The majority of the 3HP doses were given at the TB clinic (82.8%) followed by home and workplace (4%) and other (10%). Among the people who were offered treatment (n = 102), those that were new diagnoses were not more likely to start compared to those that had been previously been diagnosed with LTBI in the past (58/78, 74% new diagnosis vs 15/24, 62.5% previous diagnosis, p = 0.67).
Table 1.
Demographic characteristics of the individuals who started 3HP treatment
| Community |
Iqaluit |
Qikiqtarjuaq |
||
|---|---|---|---|---|
| Total number of individuals who started 3HP | 73 | 115 | ||
| Sex | Male | 40 (55%) | 74 (64.4%) | |
| Female | 33 (45%) | 41 (35.6%) | ||
| Ethnicity | Canadian Born Indigenous (Inuit) | 50 (69%) | 113 (98.3%) | |
| Canadian Born Indigenous (Non-Inuit) | 1(1%) | 2(1.7%) | ||
| Canadian Born Non-Indigenous | 13 (18%) | 0 | ||
| Foreign Born | 9 (12%) | 0 | ||
| Age (years) | Mean and range | 31 (2–60) | 28(1–61) | |
| Body mass index (kg/m2) | Mean and range | 27.8 (12.9–40.2) |
25.6 (15.5–43.9) |
|
| Reason for testing | Contact of an active case | 29 (40%) | - | |
| Passive walk in to clinic | 20(27%) | - | ||
| Employment screen | 21(29%) | - | ||
| School screen | 3(4%) | - | ||
| Community wide screen | 0 | 115 (100%) | ||
| Alcohol | Mean and range of drinks* per week | 7.69 (0–36) | - | |
| Cannabis | Mean joints per week | 3.3 (0–8) | - | |
| Smoking | Mean and range cigarettes per day | 10 (1–25) | - | |
*one drink = 1 beer = 1 oz of hard liquor = 1 glass of wine.
Overall, 60 out of 73 participants in Iqaluit that started treatment completed treatment (82.2%) (Figure 1, Table 2) using the previously established completion definition [10]. Four people who started treatment were deemed not eligible after starting (2 children on window prophylaxis with a second negative TST, 1 with active TB disease and 1 with severe alcoholism) therefore did not have the chance to complete and were removed from further analysis. In Iqaluit 72% agreed to treatment with 3HP compared to 78.5% who agreed to start standard INH pre-study (p = 0.24) (Table 2). Although statistical significance was not reached, more people who started treatment completed treatment in the 3HP group (Iqaluit, 60/73 (82.2%) and Qikiqtarjuaq, 89/115 (77.4%)) than in the historical control 9INH group (306/420 = 72.9%) (p = 0.2) (Table 2).
Figure 1.
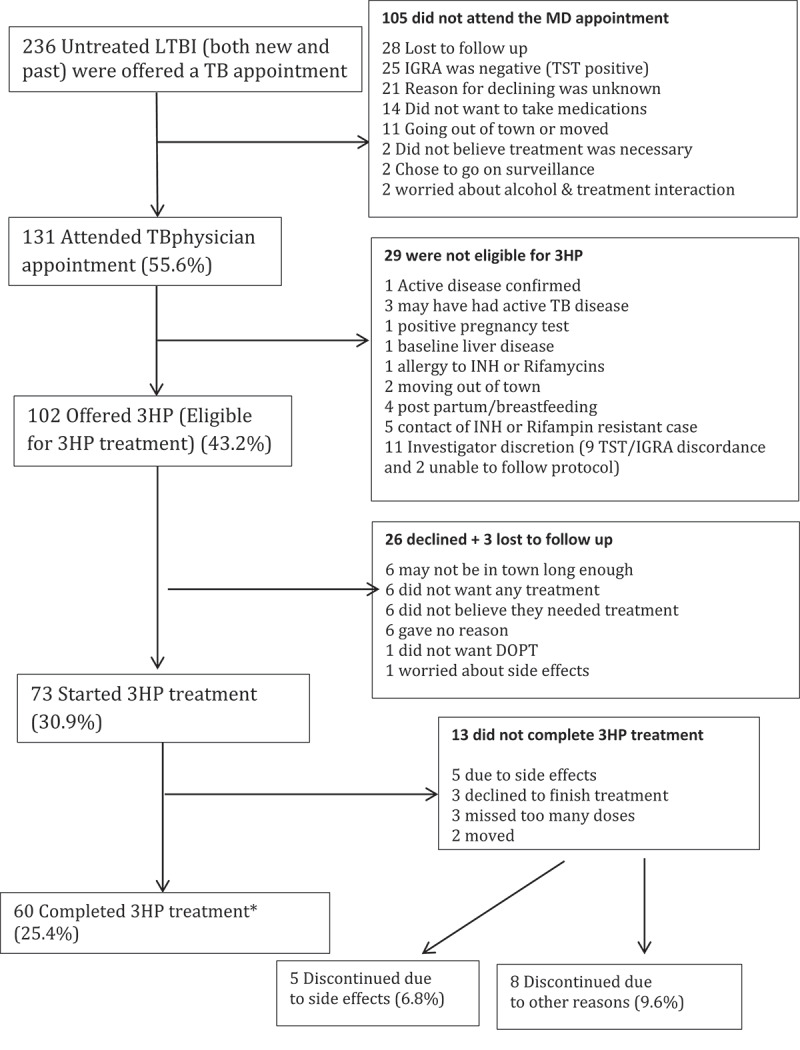
The latent TB cascade of care in Iqaluit from June 2016 to June 2018
*Completion definition used was a minimum of 90% of the doses (11/12) given over a maximum of 16 weeks.
Table 2.
Proportion of people who were offered, started and completed LTBI treatment given directly observed stratified on different completion definitions*
| Community/Regimen |
||||
|---|---|---|---|---|
| Iqaluit 9INH § | Iqaluit 3HP | Qikiqtarjuaq 3HP | p value | |
| Started/Offered | 78.5% (420/535) | 71.5% (73/102) | 80% (115/145) | 0.27 |
| Completion*/Started** | ||||
| ≥80% of doses | 76.0% (319/420) | 82.2% (60/73) | 77.3%(89/115) | 0.50 |
| ≥90% of doses | 72.9% (306/420) | 82.2% (60/73) | 77.3%(89/115) | 0.19 |
| 100% of doses | 65.2% (274/420) | 72.6% (53/73) | 73.9% (85/115) | 0.11 |
§ Data collected among all patients starting INH in Iqaluit between January 2010–March 2016.
*Completion of 9 INH of twice weekly doses within a maximum of 12 months and completion of 3HP once weekly doses within a maximum of 16 weeks.
**Started means at least one dose of medication taken.
The most common adverse events in Iqaluit (Figure 2) were muscle aches, fatigue and headache. Most of the adverse events were grade 1 events which were associated with mild discomfort but no disruption of normal daily activity. Five participants did not complete 3HP due to adverse events, they included 3 with grade 3 events, 1 with a grade 2 event and 1 with a grade 1 event. No participants experienced grade 4 or 5 levels of severity. Overall, 13 people did not complete treatment. Eight people stopped treatment due to non-medical issues and the other five were related to adverse events (Figure 1). No women became pregnant while on 3HP.
Figure 2.
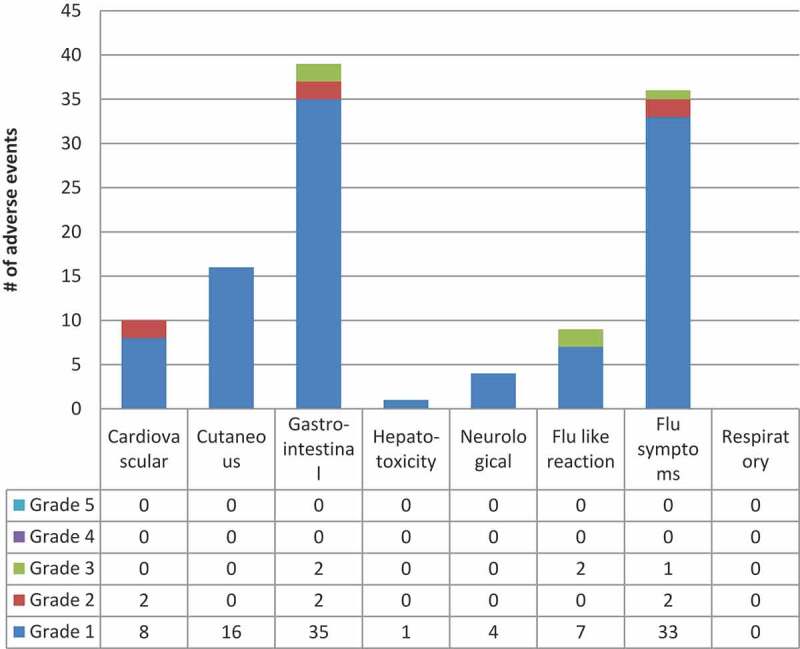
3HP related or possibly related adverse events by system & grade in Iqaluit
* Includes all Adverse Events reported by participants deemed as “related” or “possibly related” to 3HP by the site investigator**Number of participants experiencing a certain severity of an adverse event with one or more doses where each participant is counted only once at the highest level of severity for that system*** Grade 1 = Mild – discomfort noticed but no disruption of normal daily activity, Grade 2 = Moderate – discomfort sufficient to reduce or affect daily activity, Grade 3 = Severe – inability to work or perform a normal daily activity, Grade 4 = Life Threatening or Disabling – represents an immediate threat to life, Grade 5 = Death – related to Adverse Eventⱡ Other related or possibly related adverse events included insomnia, back pain and erectile dysfunction (All Grade 1) System definitions can be found in the supplement under, classification of adverse events by system.
The univariable model (Table 3) showed that abstinence from alcohol (OR 13.33, 95% CI, 2.27–78.20) and not being on concomitant medication was associated with increased completion (OR 7.19, 95% CI, 1.47–35.30). None of the variables achieved significance in the multivariable model.
Table 3.
Univariable and multivariable analysis of key factors associated with completing 3HP treatment in Iqaluit (n = 73)
| Factor | Completed n/73 (%) | Univariate analysis odds ratio (95% CI) |
Multivariate analysis odds ratio (95% CI) |
|---|---|---|---|
| Age (10 year increment) | 60(82.2%) | 0.96(0.91,1) | 0.99(0.94,1.04) |
| Sex | |||
| Male | 35(87.5%) | 2.24(0.66,7.66) | 1.16(0.26,5.15) |
| Female | 25(75.8%) | Reference | |
| Ethnicity | |||
| Inuit | 40(80.0%) | 0.6(0.15,2.43) | 1.13(0.18,7.05) |
| Non-Inuit | 20(87.0%) | Reference | |
| Employment | |||
| Yes | 33(82.5%) | 1.05(0.32,3.49) | 1.50(0.30,7.65) |
| No | 27(81.8%) | Reference | |
| Alcohol Use(cage score) | |||
| No alcohol use | 32(88.9%) | 13.33(2.27,78.20) | 9.45(0.91,98.0) |
| Cage score <2 | 25(86.2%) | 10.42(1.76,61.67) | 6.93(0.76,62.76) |
| cage score ≥2* | 3(37.5%) | Reference | |
| Cannabis use | |||
| No | 46(82.1%) | 0.99(0.24,4.09) | 0.35(0.05,2.64) |
| Yes | 14(82.4%) | Reference | |
| Smoking | |||
| No | 31(91.2%) | 3.56(0.89,14.25) | 2.66(0.50,14.16) |
| Yes | 29(74.4%) | Reference | |
| Treatment reason | |||
| Contact screening | 27(93.1%) | Reference | |
| Employer screening | 16(76.2%) | 0.27(0.05,1.42) | 0.5(0.07,3.43) |
| School screening | 3(100%) | 0.64(0.02,24.87) | 0.55(0.01,33.01) |
| Walk in to clinic | 14(70.0%) | 0.20(0.04,1.03) | 0.22(0.04,1.31) |
| Concomitant medications | |||
| No | 34(94.4%) | 7.19(1.47,35.30) | 4.68(0.94,23.28) |
| Yes | 26(70.3%) | Reference |
*A total score of 2 or greater is considered clinically significant for substance abuse disorder. [28]
Ethnicity (Inuit versus non-Inuit), employment (paid employment, yes or no), alcohol (no alcohol, Cage score <2 versus ≥2), cannabis (no versus yes), smoking (no versus yes), treatment reason (contact, walk in, school screen, employer screen), concomitant medications (no versus yes).
Among participants in the Qikiqtarjuaq programmatic role out (Figures 3 and 4) who started 3HP, the mean age was 28, almost all of the participants were Inuit and all were screened as part of a community-wide screening program (Table 1). During the programmatic role out of 3HP in Qikiqtarjuaq 80% of participants who were offered treatment started treatment and 77.4% of those that started completed treatment (using the ≥90% of doses in a maximum of 16 weeks definition) (Table 3). Adverse events were all grade 1 or 2, 15 participants did not complete 3HP treatment as a result of an adverse event (Figures 3 and 4). Of those, seven were due to the flu-like reaction [26]. Of the 15 that did not complete due to adverse events, 7 were grade 1 severity, 7 were grade 2 severity, one was not graded and 8 occurred after the first dose.
Figure 3.
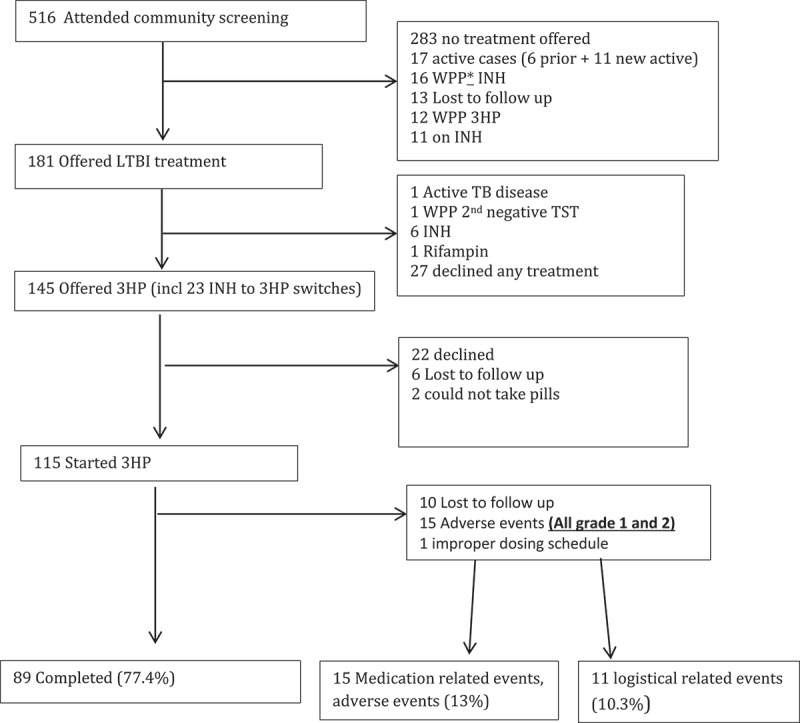
The latent TB cascade of care in Qikiqtarjuaq from February to August 2018
*WPP = window period prophylaxis (paediatrics).
Figure 4.
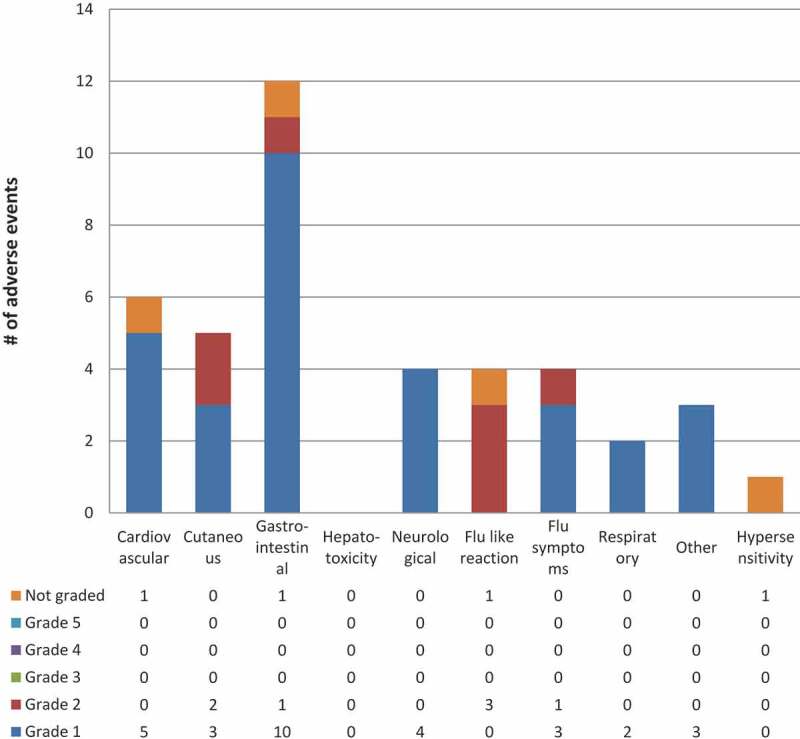
3HP related or possibly related adverse events by system & grade in Qikiqtarjuaq programmatic roll out
* Includes all Adverse Events reported by participants deemed as “related” or “possibly related” to 3HP by the health team**Number of participants experiencing a certain severity of an adverse event with one or more doses where each participant is counted only once at the highest level of severity for that system*** Grade 1 = Mild – discomfort noticed but no disruption of normal daily activity, Grade 2 = Moderate – discomfort sufficient to reduce or affect daily activity, Grade 3 = Severe – inability to work or perform a normal daily activity, Grade 4 = Life Threatening or Disabling – represents an immediate threat to life, Grade 5 = Death – related to Adverse EventSystem definitions can be found in the supplement under, classification of adverse events by system.
Discussion
The use of 3HP for the treatment of LTBI among this predominantly Inuit population in a remote region of Canada was feasible, well accepted and completion rates were similar to the large randomised controlled studies and phase 4 studies done in major U.S cities. In the original 3HP RCT [10], the completion rate was 82.1% in the 3HP arm and in the phase 4 study done in several US urban cities, the completion rate was 87.2% [13]. In the present study, the completion rate was comparable at 82.2% in Iqaluit and 77.4% in Qikiqtarjuaq using the same regimen and outcome definitions. Most importantly, the adverse events were comparable with previous studies and were mild and did not affect overall completion rates.
Although statistical significance was not reached, more people completed treatment in the 3HP group than the historical control 9INH group (82.2% versus 72.9%, p = 0.2). This may have been a result of the baseline completion rate in Iqaluit of 72.9% being significantly above the rates found in the meta-analysis of completions from around the world of 62% [6]. Use of 3HP did not increase the proportion of people who agreed to start TB prophylaxis compared to historical 9INH rates. A study done in New York City also found that 3HP did not increase the number of people starting TB treatment when compared to 9INH [27]. The fact that rifapentine was not Health Canada approved during the study may have had some influence on the number of participants that ended up starting 3HP. In addition, patients had to commit to at least 2 hours at the study entry to be informed about the study, review consent with the nurse and physician which may have been a deterrent for some participants.
The adverse events noted in this study were mild and were consistent with previous studies. Rifapentine is known to cause a flu-like illness in some patients and this was the most common side effect observed in our study. These reactions were mostly grade 1 severity however three people had grade 3 reactions and had to stop 3HP because of it. These participants did not experience any long-term sequelae from this adverse event. We observed that the flu-like illness was indistinguishable from what some people experience after the influenza vaccine and therefore we recommend that people get vaccinated for influenza before starting 3HP or at least not on the same day as receiving 3HP.
The Qikiqtarjuaq program roll out of 3HP provided a real-world front-line pragmatic approach. However, the roll out was limited by the fact that the front-line team providing 3HP received some training but had no experience using it. This may have led to treatments being stopped earlier than would have been done in common practice resulting in the decreased number of completions (82% vs 77%). The number of grade 1 adverse events that led to the discontinuation of treatment in Qikiqtarjuaq was 7 compared to only one in Iqaluit. This highlights the fact that front-line nurses and doctors need to have comprehensive training on the use of this regimen and need telephone support at a minimum, to ask questions regarding starting treatment and discontinuation due to adverse events.
Completions aside, given that the side effect profile was generally favourable and comparable to the international literature in terms of the type and frequency of adverse events experienced by this predominantly Inuit population, in a region that has severe human resource shortages and high turnover of health workers, 3HP offers a significant reduction in the workload for the staff. Compared to 9 H which requires 72 doses given DOPT, the 12-dose 3HP regimen offers an 85% reduction in the number of DOPT doses that need to be given. Further, the reduction of doses significantly decreases the inconvenience and time commitment for patients. Our group is currently completing a 3HP cost-effectiveness study in the region.
The limitations of this study include the fact that the comparator group was a historical control which may have introduced temporal confounding/bias. In addition, the study conditions in Iqaluit also increase the chance of producing a higher than normal delivery of care which may have resulted in higher completion rates. However, the roll out in Qikiqtarjuaq provided a program level pragmatic picture of the uptake and completion of 3HP, and the completion rates although slightly decreased were comparable to those seen in Iqaluit under strict study protocol. In addition, even though historical controls were used, no other major TB interventions or programs were introduced during the study period in Iqaluit.
The strengths of this study lie in the fact that it is the first study in Canada of the implementation of 3HP in a primarily Inuit population in a remote region of Canada. In addition, this study was carried out in a region that faces the greatest incidence of TB where new treatments and technologies are often the last place to be implemented due to its remoteness and lack of human infrastructure to run new programs. Further, this study provided a procurement algorithm and clinical protocol specific for remote arctic regions developed under research conditions and honed-in a programmatic setting.
Conclusions
This is the first study to demonstrate the feasibility and safety profile of 3HP for the treatment of LTBI in Nunavut, Canada. The number of persons that completed the regimen in this remote arctic setting with a predominantly Inuit population was improved compared with a historical cohort from Iqaluit and was comparable to completion rates found in large randomised controlled trials.
Total word count = 2752 (not including abstract)
Supplementary Material
Acknowledgments
The authors would like to thank the following individuals for their help with this study: Jean Allen, Kim Barker, Monica Taljaard, Andrea Schertzer, Dana Carr, Joan Fraser and Kristine Hutchison.
Funding Statement
Public Health Agency of Canada
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Data
Supplemental material for this article can be accessed here.
References
- [1].Vachon JGV, Siu W.. Tuberculosis in Canada, 2016. Can Commun Dis Rep. 2018;44(3/4):75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Inuit Tapariit Kanatami . TB elimination among Inuit communities. 2018. Available from: https://www.itk.ca/the-government-of-canada-and-inuit-tapiriit-kanatami-commit-to-eliminating-tuberculosis-across-inuit-nunangat-by-2030/
- [3].Dye C, Glaziou P, Floyd K, et al. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–286. [DOI] [PubMed] [Google Scholar]
- [4].Dehghani K, Lan Z, Li P, et al. Determinants of tuberculosis trends in six Indigenous populations of the USA, Canada, and Greenland from 1960 to 2014: a population-based study. Lancet Public Health. 2018;3(3):e133–e42. . [DOI] [PubMed] [Google Scholar]
- [5].Liu Y, Birch S, Newbold KB, et al. Barriers to treatment adherence for individuals with latent tuberculosis infection: A systematic search and narrative synthesis of the literature. Int J Health Plann Manage. 2018;33(2):e416–e33. [DOI] [PubMed] [Google Scholar]
- [6].Alsdurf H, Hill PC, Matteelli A, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–1278. [DOI] [PubMed] [Google Scholar]
- [7].Inuit Tapariit Kanatami . Inuit statistical profile. 2018. Available from: https://www.google.ca/search?q=Inuit+Statistical+Profile+2018&rlz=1C1NHXL_enCA721CA721&oq=Inuit+Statistical+Profile+2018&aqs=chrome.69i57.1406j0j7&sourceid=chrome¡UTF-8
- [8].Patterson M, Finn S, Barker K.. Addressing tuberculosis among Inuit in Canada. Can Commun Dis Rep. 2018;44(3/4):82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Health Organization . Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf;jsessionid=E95EF4C8614F4FD6C7527D831402EEF4?sequence=1 [PubMed]
- [10].Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–2166. . [DOI] [PubMed] [Google Scholar]
- [11].Villarino ME, Scott NA, Weis SE, et al. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015;169(3):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun HY, Huang YW, Huang WC, et al. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: A multicentre randomised controlled trial in Taiwan. Tuberculosis (Edinb). 2018;111:121–126. [DOI] [PubMed] [Google Scholar]
- [13].Sandul AL, Nwana N, Holcombe JM, et al. High rate of treatment completion in program settings with 12-dose weekly isoniazid and rifapentine for latent mycobacterium tuberculosis infection. Clin Infect Dis. 2017;65(7):1085–1093. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schmit KM, Lobato MN, Lang SG, et al. High completion rate for 12 weekly doses of isoniazid and rifapentine as treatment for latent mycobacterium tuberculosis infection in the federal bureau of prisons. J Public Health Manag Pract. 2019;25(2):E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nwana N, Marks SM, Lan E, et al. Treatment of latent mycobacterium tuberculosis infection with 12 once weekly directly-observed doses of isoniazid and rifapentine among persons experiencing homelessness. PLoS One. 2019;14(3):e0213524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schein YL, Madebo T, Andersen HE, et al. Treatment completion for latent tuberculosis infection in Norway: a prospective cohort study. BMC Infect Dis. 2018;18(1):587. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Froberg G, Jansson L, Bruchfeld J. Treatment of latent tuberculosis with 12 weeks isoniazid/rifapentine in clinical practice. Eur Respir J. 2019;53:2. [DOI] [PubMed] [Google Scholar]
- [18].Macaraig MM, Jalees M, Lam C, et al. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuberc Lung Dis. 2018;22(11):1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pease C, Hutton B, Yazdi F, et al. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: a systematic review with network meta-analyses. BMC Infect Dis. 2017;17(1):265. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pease C, Hutton B, Yazdi F, et al. A systematic review of adverse events of rifapentine and isoniazid compared to other treatments for latent tuberculosis infection. Pharmacoepidemiol Drug Saf. 2018;27(6):557–566. . [DOI] [PubMed] [Google Scholar]
- [21].Borisov AS1, Bamrah Morris S1, Njie GJ1, Winston CA1, Burton D1, Goldberg S1, Yelk Woodruff R1, Allen L1, LoBue P1, Vernon A1. Update of Recommendations for Use of Once-Weekly Isoniazid-Rifapentine Regimen to Treat Latent Mycobacterium tuberculosis Infection. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):723–726. doi: 10.15585/mmwr.mm6725a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Government of Canada . List of drugs for an urgent public health need 2018. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/access-drugs-exceptional-circumstances/list-drugs-urgent-public-health-need.html
- [23].Pease C, Zwerling A, Mallick R, et al. The latent tuberculosis infection cascade of care in Iqaluit, Nunavut, 2012-2016. BMC Infect Dis. 2019;19(1):890. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nunavut Bureau of Statistics . Population Estimates 2018. Available from: http://www.stats.gov.nu.ca/en/Population%20estimate.aspx
- [25].Ramsay CR, Matowe L, Grilli R, et al. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19(4):613–623. [DOI] [PubMed] [Google Scholar]
- [26].Sterling TR, Moro RN, Borisov AS, et al. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT tuberculosis study. Clin Infect Dis. 2015;61(4):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stennis NL, Burzynski JN, Herbert C, et al. Treatment for tuberculosis infection with 3 months of isoniazid and rifapentine in New York City health department clinics. Clin Infect Dis. 2016;62(1):53–59. [DOI] [PubMed] [Google Scholar]
- [28].Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905–1907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Inuit Tapariit Kanatami . TB elimination among Inuit communities. 2018. Available from: https://www.itk.ca/the-government-of-canada-and-inuit-tapiriit-kanatami-commit-to-eliminating-tuberculosis-across-inuit-nunangat-by-2030/
- Inuit Tapariit Kanatami . Inuit statistical profile. 2018. Available from: https://www.google.ca/search?q=Inuit+Statistical+Profile+2018&rlz=1C1NHXL_enCA721CA721&oq=Inuit+Statistical+Profile+2018&aqs=chrome.69i57.1406j0j7&sourceid=chrome¡UTF-8
- World Health Organization . Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf;jsessionid=E95EF4C8614F4FD6C7527D831402EEF4?sequence=1 [PubMed]
- Government of Canada . List of drugs for an urgent public health need 2018. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/access-drugs-exceptional-circumstances/list-drugs-urgent-public-health-need.html
- Nunavut Bureau of Statistics . Population Estimates 2018. Available from: http://www.stats.gov.nu.ca/en/Population%20estimate.aspx


