Figure 4.
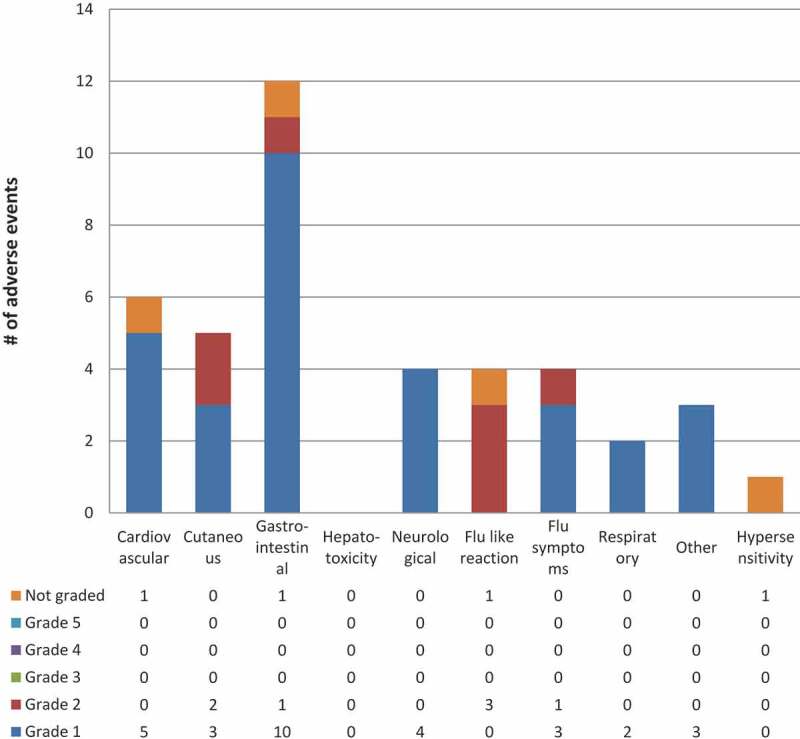
3HP related or possibly related adverse events by system & grade in Qikiqtarjuaq programmatic roll out
* Includes all Adverse Events reported by participants deemed as “related” or “possibly related” to 3HP by the health team**Number of participants experiencing a certain severity of an adverse event with one or more doses where each participant is counted only once at the highest level of severity for that system*** Grade 1 = Mild – discomfort noticed but no disruption of normal daily activity, Grade 2 = Moderate – discomfort sufficient to reduce or affect daily activity, Grade 3 = Severe – inability to work or perform a normal daily activity, Grade 4 = Life Threatening or Disabling – represents an immediate threat to life, Grade 5 = Death – related to Adverse EventSystem definitions can be found in the supplement under, classification of adverse events by system.
