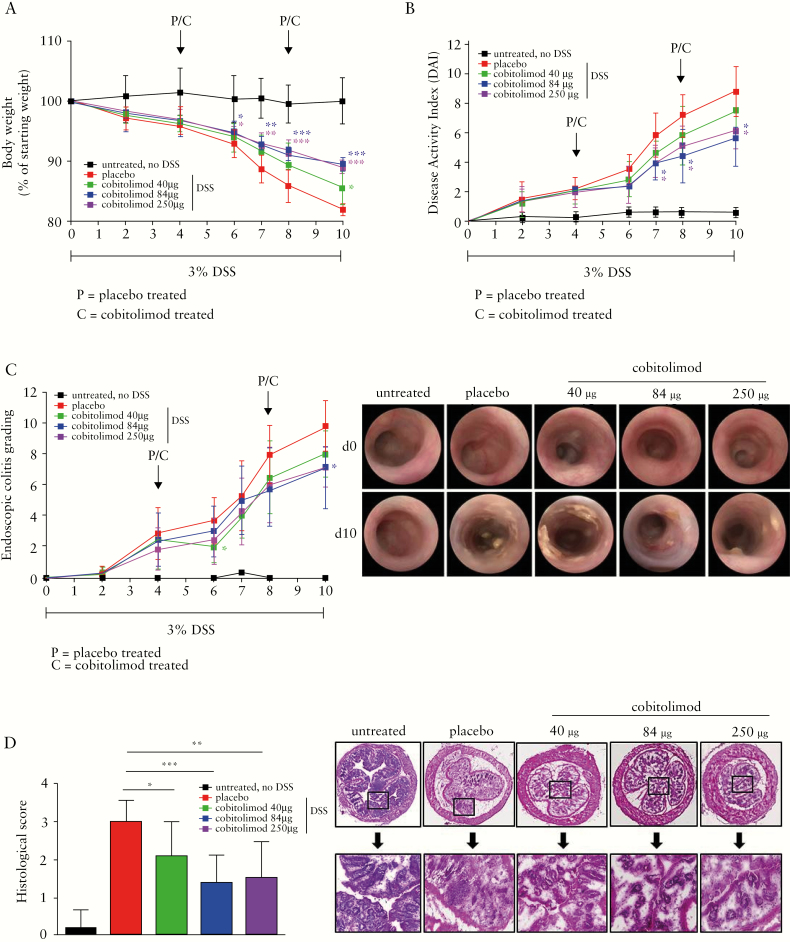
Figure 2.
Impact of cobitolimod treatment on clinical, endoscopic and histological disease activity in the experimental mouse model of DSS-induced colitis. 3% DSS was added for 10 days to the drinking water of female Balb/c mice. A control group [no DSS] was also part of the experimental set-up [n = 3]. Cobitolimod [40, 84 or 250 µg] or placebo, was rectally administered on days 4 and 8 [n = 7 mice per group] to DSS-treated mice. [A] Body weight was monitored on days 0, 2, 4, 6, 7, 8 and 10. [B] Disease activity index [DAI] is the combined score of body weight loss compared to initial body weight, stool consistency and visible blood in faeces. The maximum score per mouse is 12. DAI was assessed on days 0, 2, 4, 6, 7, 8 and 10. [C] Endoscopic colitis grade consists of the following five parameters: thickening of the colon, change of the normal vascular pattern, presence of fibrin, mucosal granularity and stool consistency. Grading [score 0–3] was performed for each parameter leading to a final accumulative score, the endoscopic colitis grading, between 0 and 15. Endoscopic grading was analysed on days 0, 2, 4, 6, 7, 8 and 10 [left panel]. Representative endoscopic examples for day 0 and day 10 for each group are shown [right panel]. [D] Histopathological analysis of intestinal sections. A histological score [0 = no inflammation, 1 = remission, 2 = mild, 3 = moderate, 4 = severe colitis] was used to quantify severity of DSS-induced colitis [left panel] in mice intestinal specimens taken at day 10. Representative intestinal H&E stainings of each group are shown [right panel]. Data represent mean values ± SD. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 comparing cobitolimod vs placebo-treated animals.