Graphical abstract
Characteristics of host-microbiota interactions in asthmatic children favor asthma attacks and may allow to define asthma endotypes.
This knowledge offers promising perspectives to treat asthmatic children using precision medicine.
Keywords: Acute asthma, Asthma exacerbation, Childhood, Virus, Microbiota, Therapeutics
Abstract
Exacerbations are a main characteristic of asthma. In childhood, the risk is increasing with severity. Exacerbations are a strong phenotypic marker, particularly of severe and therapy-resistant asthma. These early-life events may influence the evolution and be involved in lung function decline.
In children, asthma attacks are facilitated by exposure to allergens and pollutants, but are mainly triggered by microbial agents. Multiple studies have assessed immune responses to viruses, and to a lesser extend bacteria, during asthma exacerbation. Research has identified impairment of innate immune responses in children, related to altered pathogen recognition, interferon release, or anti-viral response. Influence of this host-microbiota dialog on the adaptive immune response may be crucial, leading to the development of biased T helper (Th)2 inflammation. These dynamic interactions may impact the presentations of asthma attacks, and have long-term consequences.
The aim of this review is to synthesize studies exploring immune mechanisms impairment against viruses and bacteria promoting asthma attacks in children. The potential influence of the nature of infectious agents and/or preexisting microbiota on the development of exacerbation is also addressed. We then discuss our understanding of how these diverse host-microbiota interactions in children may account for the heterogeneity of endotypes and clinical presentations. Finally, improving the knowledge of the pathophysiological processes induced by infections has led to offer new opportunities for the development of preventive or curative therapeutics for acute asthma. A better definition of asthma endotypes associated with precision medicine might lead to substantial progress in the management of severe childhood asthma.
1. Introduction
Childhood asthma is a major public health issue in industrialized countries. Development and progression of the disease is marked by exacerbations, also called asthma attacks, which are one of the most prevalent causes of hospitalization in children with consequently significant costs worldwide [1], [2], [3]. They constitute key events in the natural history of childhood asthma and are major markers of its heterogeneity [4]. Mechanisms leading to asthma attacks are not fully understood and may differ between children, according to their age range or phenotype.
Pediatric literature on asthma usually distinguishes preschool recurrent wheeze/also called preschool asthma in children aged 5 years and younger; from school-age children asthma, in those aged 6–11 years; and adolescent asthma, in those aged 12–17 years [3], [5], [6]. During preschool years, these events can be isolated, with few or no interval symptoms, and their persistence despite conventional maintenance treatment, such as inhaled corticosteroids (ICS), is a marker of severity [6], [7]. Although viral infections are the main triggers of acute asthma, early and multiple allergic sensitization is a strong risk factor of presenting severe and frequent exacerbations [7], [8]. In school-age children and adolescents, acute asthma attacks are usually less frequent, and not only triggered by lung infections, but also by exposure to allergens and pollutants [3], [9], [10]. Exacerbations rates increase with the severity of the disease, which is defined according to treatment pressure to obtain asthma control [4], [5]. Repetition of frequent and severe episodes under high doses of ICS associated with another maintenance treatment (mainly long acting beta2 agonists) is a criteria of severe asthma in these age-groups [11], [12], as demonstrated in pediatric asthma cohorts [13], [14]. Additionally, it is now recognized that patients prone to exacerbations constitute a distinct susceptibility phenotype, possibly associated with different endotypes, including specific airway inflammation patterns, or inflammatory mechanisms in response to infectious agents [15]. In some individuals, the severity of the disease and propensity to develop acute asthma may also vary throughout the course of childhood [16]. These early-life events can influence the long-term evolution at adult-age, in terms of asthma persistence, phenotype and alterations of lung function, that may lead to early chronic obstructive pulmonary disease (COPD) [17]. Early interventions could modify the natural history of the disease. However, in children with severe asthma, conventional maintenance therapies may be insufficient to prevent asthma attacks [7], [18], and repeated or prolonged oral corticosteroids are associated with many side effects, such as defective growth [19]. New treatment strategies are now needed. Interestingly, some studies have shown the efficacy of biologics in pediatric asthma, mainly on severe exacerbations, and suggested disease-modifying properties of some of them, making it a particularly attractive strategy in children [20].
Viruses are closely linked to wheezing illnesses during early childhood [21]. Respiratory syncytial virus (RSV), and rhinoviruses (RV), both RNA viruses, are among the most common causes of lower respiratory tract infections, i.e. bronchiolitis and/or pneumonia, with a predominance of RSV in the first 12 months of life and then RV in the following years [22]. Birth cohort studies and other epidemiological studies have shown a strong association between early RV-induced wheeze, and to a lesser extent, RSV-induced wheeze, and subsequent development of childhood asthma [23], [24], [25], [26]. Moreover, pediatric asthma attacks are mainly triggered by respiratory viruses, particularly RV, both in the preschool and school-age [10], [27], [28], [29], [30], [31]. The question of whether asthma-associated underlying inflammation promotes the pathogenic effect of viral infection or whether the virus induces exaggerated inflammation is still under debate and has been the subject of many research studies over the past 25 years.
Alongside viruses, there is also a growing interest on the impact of bacteria in the onset of asthma attacks and the perpetuation of inflammation. Neonatal airway colonization with bacteria including Haemophilus influenzae, Moraxella catarrhalis and/or Streptococcus pneumoniae, has been associated with persistence of asthma [32]. Indeed, Kloepfer, et al. have shown frequent viral and bacterial co-infection in asthmatic children aged 2–18 years [33]. It is well known that viral infection can predispose to bacterial secondary infection. A few studies have begun to describe inflammatory phenotypes linked with altered microbiota, known as dysbiosis, within the airways [34], [35]. Interactions between viruses and bacteria, personal risk factors (e.g. genetic background and atopy), and environmental exposures may promote more severe acute asthma episodes that will influence the progression of asthma [21]. However, the precise role of each of these factors and their interplay with the host immune defenses remains to be elucidated.
The aim of this review was to synthesize studies exploring human innate immune mechanisms responses against viruses and bacteria during asthma attacks and to provide hypotheses to decipher how they may contribute to the phenotypes observed in childhood asthma. We will then discuss how therapeutic strategies targeting these pathways may improve the management of acute asthma in children.
2. Impact of viruses on the onset of asthma attacks: chicken or egg?
Up to now, mechanisms explaining susceptibility to develop acute asthma upon respiratory viruses, in particular RV and to a lesser extend RSV, in asthmatic children prone to exacerbation are not fully understood. As innate immune cells play a central role in the onset of anti-viral defenses, they have been the focus of many studies in pediatric and adult asthma, conducted both ex-vivo and in-vivo, contributing to the identification of new asthma endotypes. The original studies presented in this review have been summarized in Table 1 .
Table 1.
Major studies (pediatric and adult population) assessing immune responses to viral infection during asthma exacerbation.
| Compartment | Study | Design and age-group | Main results | |
|---|---|---|---|---|
| PRR | In vitro and ex-vivo | |||
| AEC | Edwards M, et al. 2013[45] | RV-A16 Children:Severe therapy resistant asthma (STRA) 11y (9-15y) vs healthy controls | In STRA: lower basal levels of TLR3, reduced RIG-I, MDA5 levels after RV stimulation; lower basal levels of TLR3, reduced RIG-I, MDA5 levels after RV stimulation No association with age, IgE levels, allergen reactivity, BAL/sputum neutrophils and eosinophils, lung function | |
| AEC | Parsons KS, et al. 2014[46] | RV-B1 Adults:asthma/Healthy controls | In asthma: no impairment of expression of MDA5, TLR3 and reduced release of CXCL-10 | |
| AEC | Contoli M, et al. 2015[59] | RV-A16 Pre-treatment with IL-4 and IL-13 prior to infection Adults:atopic rhinitis/non-atopic healthy | In atopic cells: impaired immune response to RV-16 in the presence of IL-4 and IL-13, through inhibition of TLR3 expression and signaling (IRF3) | |
| AEC | Moskwa S, et al. 2018[61] | PIV-3/RV-B1 Adults:atopic asthma/non-atopic asthma/healthy controls | Atopic asthma: After PIV3 infection: higher IFN-λ1, IFN- α, IFN- β and IRF7 expressions After RV1B infection: IFN- β higher than in non-atopic asthmatics | |
| AM | Rupani H, et al. 2016[47] | RV-A16 Pre-treatment with Imiquimod (TLR7 agonist) Adults:severe asthma/Healthy controls | In severe asthma: reduced TLR7 expression, inverse correlation with number of exacerbations Increased levels of 3 microRNAs (miR-150, miR-152, and miR-375) Restored TLR7 expression after blocking these microRNAs with anti-miRNA oligonucleotides | |
| BAL cells PBMC | Sykes A, et al. 2012[48] | RV-A16 Adults:mild-to-moderate allergic asthma vs healthy controls | In asthma: no impairment of expression of PRR (TLR, RNA-helicases), their adaptor proteins, transcription factors downstream | |
| In vivo | ||||
| Sputum Serum PBMC | Deschildre A, et al. 2017[49] | School-age children 8.9 y (6-16y)Atopic asthma: virus vs no virus Severe exacerbation (64% virus, 51% RV)/steady state (8 weeks later) (25% virus, 11% RV) 24% virus at both time | Virus at exacerbation: similar TLR3, RIGI-I and MDA-5 expression on blood monocytes and DC, similar levels of IFN-β, IFN-γ, IFN-λ1 in sputum and plasma, higher airway IL-5 and eosinophils counts than patients with negative viral PCR Virus at both time: modification in PRR expression/function on PBMC, higher airway neutrophilic inflammation at steady state | |
| Bronchial biopsies | Zhu J, et al. 2019[67] | In vivo inoculation - RV-A16 Young adults:atopic asthma (23 y ± 1.4) vs non-atopic control subjects (27 y ± 2.3) | In asthma: PRR expression (TLR3, MDA5, RIG-I) not deficient at baseline; and induced after RV both in epithelium and sub-epithelium | |
| Type I and type III IFN | In vitro | |||
| AEC | Wark PA, et al. 2005[60] | RV-A16 Adults:Asthma vs healthy controls | In asthma: Impaired IFN-β, late virus release and late cell lysis, impaired apoptotic response | |
| AEC | Edwards M, et al. 2013[45] | RV-A16 Children:Severe therapy resistant asthma (STRA) 11y (9-15y), vs healthy controls | In STRA: IFN-β, IFN-λ1, IFN-λ2/3 mRNA levels lower and RV virus load higher No association with age, IgE levels, allergen reactivity, BAL/sputum neutrophils and eosinophils, lung function | |
| AEC | Gielen V, et al. 2015[57] | RV-B1/RV-A16 Pre-treatment with IL-4/IL-13 Children:STRA 11y (9-15y), vs healthy controls | In STRA: SOCS1 expression increased and related to IFN deficiency, increased viral replication Suppression of RV-induced IFN promoter activation in AEC by SOCS1, dependent on nuclear translocation | |
| AEC | Parsons KS, et al. 2014[46] | RV-B1 Adults:asthma/Healthy controls | In asthma: reduced release of IL-6, CXCL-8 and IFN-λ in response to RV, reduced release of CXCL-10 | |
| AEC | Kicic A, et al. 2016[58] | RV-B1/RV-B14 Children:Asthma 8.2 y (2.6–14.8) vs healthy children 8.4 y (3.2–15.6) | In asthma: greater virus proliferation and release, reduced apoptosis and wound repair (RV-B1), lower IFN-β, higher inflammatory cytokine production Addition of IFN-β: restored apoptosis, suppressed virus replication and improved repair of AEC from asthmatics, no effect on inflammatory cytokine production | |
| AM | Rupani H, et al. 2016[47] | RV-A16 Pre-treatment with Imiquimod (TLR7) Adults:severe asthma/Healthy controls | In severe asthma: reduced IFN responses Increased levels of 3 microRNAs (miR-150, miR-152, and miR-375): Blocking these microRNAs with anti-miRNA oligonucleotides increased IFN production | |
| BAL cells | Sykes A, et al. 2012[48] | RV-A16 Adults:mild-to-moderate allergic asthma vs healthy | In asthma: induction of type I IFN delayed and deficient, associated with airway hyperresponsiveness | |
| Bronchial biopsy specimens | Baraldo S, et al. 2012[56] | RV-A16 Children, 5y ± 0.5: asthma, atopy/Asthma, no atopy/Atopy, no asthma/No atopy nor asthma (Controls) | In all groups vs controls: reduced type I and III IFN production, increased RV viral RNA IFN inversely correlated with airway eosinophils, IL-4, epithelial damage, total IgE (type III IFN) | |
| Whole blood cultures | Bufe A, et al. 2002[62] | NDV Children:Allergic asthma/non-allergic asthma/allergic rhinitis/healthy control | In allergic asthma: lower virus-induced IFN-α in allergic asthma/ rhinitis: higher production of IFN-γ | |
| PBMC | Gehlhar K, et al. 2006[63] | RSV-1A/NDV Adults:Allergic asthma vs healthy controls | In allergic asthma: significant reduction of virus-induced IFN-α release, independent of virus used; no influence of medication (cortico-steroids) | |
| PBMC | Iikura K, et al. 2011[64] | RV-B14Wheeze/healthy; Young children: 2-6y; Youth group: 7–19 y; Adult group: ≥ 20y | Asthma (youth group): lower IFN-α production Asthma (youth group and adults): lower IL-6, TNF-α, IL-10 and sFasL productions Wheeze (young children): lower IL-10 associated with persistent wheeze at 2 y follow-up | |
| PBMC | Sykes A, et al. 2012[48] | RV-A16 Adults:mild-to-moderate allergic asthma vs healthy controls | In asthma: no impairement of type I IFN in PBMC, as opposed to BAL cells | |
| PBMC | Durrani S, et al. 2012[65] | RV-A16 Children 10–12 y:allergic asthma/non-allergic asthma/airborne sensitization without asthma/healthy controls. | Allergic asthma: lower IFN-responses after cross-linking FcεRI (vs sensitization and vs controls); higher surface expression of FcεRI on pDC and mDC Inverse relationship between total IgE and diminished IFN secretion, only when cross-linking of FcεRI | |
| PBMC | Simpson J, et al. 2016[66] | RV-B1 Adults:asthma, 76% atopic. Sputum phenotypes: eosinophilic/neutrophilic/paucigranulocytic/granulocytic | In neutrophilic asthma: significantly less IFN-α than PBMC from eosinophilic and paucigranulocytic asthma after RV; IFN-α inversely correlated with serum IL-6, sputum IL-1β Sputum neutrophils and dose of inhaled corticosteroids independent predictors of reduced IFN-α | |
| Type I and type III IFN | In vivo | |||
| Bronchial biopsies | Zhu J, et al. 2019[67] | In vivo inoculation - RV-A16 Young adults: atopic asthma (23 y ± 1.4) vs non-atopic control subjects (27 y ± 2.3) | In asthma: IFNα/β deficiency in epithelium, at baseline, day 4 and week 6 post-inoculation; correlated with viral load, and clinical severity Sub-epithelium: lower frequencies of monocytes/macrophages expressing IFNα/β after RV infection in sub-epithelium; subepithelial neutrophils were the source of IFNα/β | |
| BAL monocytes and AM | Contoli M, et al. 2006[71] | In vivo inoculation - RV-A16 Adults:severe asthma (ex vivo)/mild asthma without steroids treatment (ex vivo and in vivo) vs healthy controls | In asthma/Ex vivo: Impaired induction and production of type III IFN-λ1 and IFN-λ2/3 in AEC and AM, impaired induction of IFN- λ upon LPS stimulation In asthma/in vivo: severity of symptoms, BAL virus load, airway inflammation, reduction of lung function inversely correlated with ex vivo production of IFN-λ | |
| Nasal washes | Miller EK, et al. 2012[70] | Natural infection: 82% virus Children 5–18 y Upper respiratory infection; Wheezing/no-wheezing RV in 56% wheezing/37% non-wheezing | Specific association between RV infection and asthma exacerbation, but no difference in virus titers, RV species and inflammatory or allergic molecules between RV + wheezing and non-wheezing children In RV + wheezing children: IFN-λ1 level higher, increased with worsening symptoms | |
| Nasal washes | Kennedy J, et al. 2014[29] | Natural infection: 74 children (4–18 y):acute wheezing (57% RV)/acute rhinitis (56% RV)/controls Inoculation (RVA-16): 24 young adults Asthma/healthy | Natural infection: No difference in viral loads and levels of IFN-λ1 between wheezing and acute rhinitis; In children with wheezing: lower levels of sICAM-1 Inoculation: No difference in viral load between groups, levels of sICAM-1 correlated with RV levels | |
| Nasal washes Induced sputum | Schwantes E, et al. 2014[68] | Natural infection Adults:asthma vs healhy 17 exacerbations : 67% RV | In asthma, at exacerbation: sputum RNA levels of IFN-α1, IFN-β1 and IFN-γ correlated with exacerbation and the peak Asthma Index, early in the course of infection, higher levels of IL-13, IL-10 | |
| Serum PBMC | Bergauer A, et al. 2017[30] | Ex vivo inoculation of RV-1B on PBMC cultures In vivo natural infection Preschool children 4-6yAsthma at baseline (54% RV)/Asthma at exacerbation (100% RV) vs healthy controls | Ex vivo: induction of STAT1/STAT2 by RV in asthma/IRF9 in controls/IRF1 in both Natural infection (baseline): No increase of IFN-α expression in RV + asthma patients compared with RV + healthy controls; increase of IFN-λ protein with RV, both in asthma patients and controls Natural infection (exacerbation): reduced serum IFN-α at exacerbation compared with RV healthy children at baseline | |
| Sputum Serum PBMC | Deschildre A, et al. 2017[49] | School-age children 8.9 y (6-16y)Atopic asthma: virus vs no virus Severe exacerbation (64% virus, 51% RV)/steady state (8 weeks later) (25% virus, 11% RV)/24% virus at both time | Virus at exacerbation: similar levels of IFN-β, IFN-γ, IFN-λ1 in sputum and plasma, higher airway IL-5 and eosinophils Virus at both time: lower levels of IFN-γ in plasma and sputum at exacerbation, higher airway neutrophilic inflammation at steady state | |
| Sputum Plasma | Lejeune S, et al. 2020[8] | Preschool children 30 mo (1y-5y)Recurrent wheeze/preschool asthma; 47% atopy; 70% prone to exacerbation (≥2 exacerbations in the previous year) Severe exacerbation (94% virus, 73% RV)/steady state (8 weeks later) (67% virus, 50% RV) | Children prone to exacerbation: lower plasma concentrations of IFN-β and CXCL10 at exacerbation, and lower plasma levels of CXCL10 at steady state | |
| Alarmins and cytokines | In vitro | |||
| Co-culture AEC and T cells | Qin L, et al. 2011[83] | RSV-A2 AEC T cells extracted from adult PBMC (healthy volunteers) | Prolonged RSV infection in AEC induced T cells differentiation in Th2 and Th17 subsets that released IL-4, IFN-γ and IL-17 induced by supernatants from RSV-infected AEC | |
| AEC | Lee HC, et al. 2012[79] | RSV-A AEC from healthy children and asthmatic children | Production of TSLP after infection of AEC with RSV, via activation of an innate signaling pathway that involved retinoic acid induced gene I, interferon promoter-stimulating factor 1, and nuclear factor-κB. Greater levels of TSLP after RSV infection in AEC from asthmatic children than from healthy children | |
| PBMC | Jurak L, et al. 2018[82] | RV-A16 and IL-33 co-exposition Adults with allergic asthma vs healthy controls | In asthmatic cells: IL-5 and IL-13 release induced by RV, enhanced by IL-33, surface protein expression of ST2 induced by IL-33 Predominant source of IL-13 release: ILC2 In healthy cells: IL-33 had no effect on IL-5 and IL-13 production | |
| In vivo | ||||
| BAL PBMC | Jackson D, et al. 2014[81] | In vivo inoculation Ex vivo study: AEC, PBMC (T cells, ILC2) Adults:mild-to-moderate asthma vs non-atopic healthy volunteers | In vivo: IL-4, IL-5, IL-13 induced by RV in BAL in asthma Type 2 cytokines and IL-33 corelate with clinical outcomes and viral load Ex vivo: IL-5 and IL-13 production directly induced by IL-33 present in RV-infected AEC supernatants by human ILC2 and T cells | |
| PBMC Serum Nasal washes | Haag P, et al. 2018[80] | Preschool children 4-6y Ex vivo study: RVB-1 inoculation In vivo:asthma (54% RV)/controls (45% RV) at baseline | Ex vivo: Induction of ILC2 genes in asthma following RV inoculation In vivo: Down regulatation of sST2 in asthma and controls by RV Up regulation of sST2 in RV- asthma patients with low levels of 25(OH)-VitD3 In asthma: direct correlation of serum sST2 with nasal IL-33 | |
| Nasopharyngeal aspirates | Yuan XH, et al. 2020[78] | Children aged less than 14yBronchiolitis or pneumonia 19.7% RV, 35 bronchiolitis and 31 pneumonia cases | RV bronchiolitis: increased IL-4/IFN-γ and decreased TNF-α/IL-10 ratios, compared with RV pneumonia | |
| Sputum Plasma | Lejeune S, et al. 2020[8] | Preschool children 30mo (1y-5y)Recurrent wheeze/preschool asthma; 47% atopy; 70% prone to exacerbation (≥2 exacerbations in the previous year) Severe exacerbation (94% virus, 73% RV)/steady state (8 weeks later) (67% virus, 50% RV) | Children prone to exacerbation: lower plasma concentrations of IFN-γ, IL-5, TNF-α, IL-10, and lower levels of IFN-γ in sputum at exacerbation At steady state, lower plasma levels of IFN-γ | |
Studies conducted in animal models, and/or without administration of viral particles (e.g. administration of TLR agonists, double-stranded RNAs) were not included in this table.
AEC: Airway epithelial cells; AM: Alveolar macrophages; BAL: broncho-alveolar lavage; CCL: C–C motif ligand; CXCL: C-X-C motif ligand; FcεRI: high-affinity IgE receptor; IgE: Immunoglobulin E; IFN: Interferon; IL: Interleukin; ILC2: type 2 innate lymphoid cells; IRF: interferon regulatory factor; mDC: myeloid dendritic cells; LPS: Lipopolysaccharides ; MDA-5: melanoma differentiation-associated protein 5; NDV: Newcastle disease virus; PBMC: peripheral blood mononuclear cells; pDC: plasmacytoid dendritic cells; PBMC: Peripheral blood mononuclear cells; PCR: Polymerase chain reaction; PIV3: parainfluenza virus type 3; PRR: pattern recognition receptor; RIGI-I: retinoic-acid inducible gene I; RSV: Respiratory syncytial virus; RV: Rhinovirus; sFasL: Serum Soluble Fas Ligand; sICAM-1: Soluble intercellular adhesion molecule-1; siRNA: small interfering RNA; SOCS1: Suppressor Of Cytokine Signaling 1; sST2: soluble suppression of tumorigenicity 2 (IL-33 receptor IL1RL1); STAT: signal transducer and activator of transcription; STRA: severe therapy resistant asthma; Th2: T helper 2; TLR: Toll like receptor; TNF: Tumor necrosis factor.
2.1. Virus recognition during asthma attack is mainly driven by airway epithelial cells
Before addressing the viral infections impact on asthma attacks, we will briefly describe the virus-induced anti-viral responses. Airway epithelial cells (AEC) are the main cellular targets of pathogens, and act as sentinel cells in response to infection ( Fig. 1 ) [36], [37], [38]. These structural cells are a central part of innate immune response [39]. They first act as a complex physicochemical barrier, via the presence of tight intercellular junctions and the muco-ciliary escalator, clearing foreign particles out. If not cleared, Pathogen Associated Molecular Patterns (PAMP) on the surface of infectious agents, will then be recognized through Pattern Recognition Receptors (PRR), in particular Toll-Like Receptors (TLR) and intracellular RNA helicases, which will trigger innate immune responses. Although AEC play an early and central role in orchestrating innate immune defenses, innate immune cells including alveolar macrophages (AM) and dendritic cells (DC), especially plasmacytoid DC (pDC), are also involved in innate immune responses against pathogens [40], [41], [42], [43], [44].
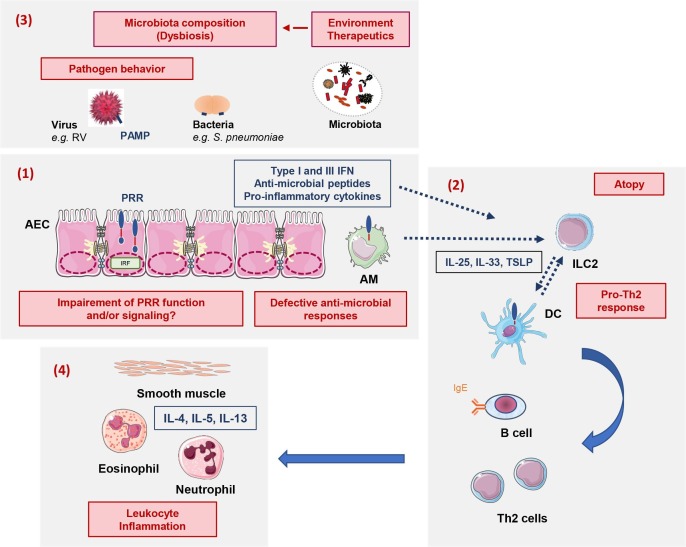
Fig. 1.
Summary of the main mechanisms favoring asthma development and involved in asthma attack. These mechanisms (in red) involve: (1) Impairment of innate immune responses; (2) Influence of the host-microbiota dialog on Th2 inflammation; (3) Pathogen characteristics; (4) Airway leukocyte inflammation. These dynamic interactions may impact the presentations of asthma attacks, and have long-term consequences. AM: Alveolar macrophages; AEC: airway epithelial cells; DC: dendritic cells; IFN: Interferon; IL: Interleukin; ILC2: type 2 innate lymphoid cells; IRF: interferon regulatory factor; PAMP: Pathogen-associated molecular pattern; PRR: pattern recognition receptor; RV: Rhinovirus; TSLP: Thymic stromal lymphopoietin.
Studies aiming to explore PRR recognition by epithelial cells and innate immune cells from asthmatic children are multiple but heterogeneous. In cultures of primary AEC from children with severe therapy resistant asthma (STRA), lower expression levels of extracellular and intracellular PRR than healthy controls were observed at baseline [45]. Expression of PRR was also decreased upon infection with RV on these cultures and in those from adult asthmatics, suggesting a persistent defect in adulthood [45], [46]. Hence, in AM from adults with severe asthma, Rupani, et al. also showed a reduction of TLR7 expression after RV infection compared with healthy subjects, with an effect potentially mediated by microRNAs [47]. Conversely, in ex vivo cultures of cells from broncho-alveolar lavages (BAL) cells and peripheral blood mononuclear cells (PBMC) from adults with mild-to-moderate asthma, no impairment of PRR expression (TLR, RNA-helicases) was observed [48]. This discrepancy was also illustrated by our in vivo real-life study conducted in 72 school-age children with allergic asthma hospitalized for severe exacerbation, associated with viral infection in 64% (RV: 51%) [49]. Whereas viral infection did not modulate PRR expression and function on PBMC as compared with non-infected asthmatic patients, a reduced expression of PRR was reported in a sub-group of children prone to reinfection outside an exacerbation (steady state 8 weeks later), and displaying neutrophilic airway inflammation away from exacerbation [10], [49]. Although present both at exacerbation and steady state, this impairment of PRR expression as well as the inflammatory profile was not associated with the clinical evolution and risk of exacerbations in the year following exacerbation [50].
Altogether, these data suggest that impairment of PRR recognition in asthmatic children could be limited to a subpopulation of patients, potentially severe, and may persist into adulthood. Rather than an altered baseline expression or synthesis of PRR, a down-regulation of the expression and activation of the co-receptors, and/or enzymes involved in signaling may be determinant. Longitudinal in vivo studies could provide more insights into these mechanisms and allow studying their dynamics during the course of the disease.
2.2. Modulation of anti-viral responses
Activation of PRR on AEC and innate immune cells will onset various signaling pathways, such as interferon regulatory factors (IRF) 3 and 7, and nuclear factor-kappa B pathway (NF-kB). This will lead to the production of anti-viral molecules such as interferons (IFN) and anti-microbial peptides, including interferon-stimulated genes (ISG) as well as cathelicidins and defensins [51]. Anti-microbial peptides have immunomodulatory activities and can alter host cellular responses. LL-37, also called human cationic antimicrobial peptide is the only human peptide of the cathelicidin family and one of the most-studied [52], [53]. In in vitro models, LL-37 has the ability to reduce virus replication, notably for RSV [53], [54]. In a population of 77 asthmatic children, Arikoglu, et al. have shown that children prone to develop acute asthma attacks displayed higher blood levels of cathelicidins and lower levels of vitamin D at baseline than children with controlled asthma, suggesting a role for anti-microbial peptides in acute asthma [55]. However, the evolution of these baseline levels during acute asthma attacks and their impact on the anti-viral and inflammatory response remain unknown.
The majority of studies conducted in asthmatic subjects have had the aim to explore anti-viral IFN response upon viral infection. They have observed a defect of production of type I IFN. In response to RV, most ex-vivo studies have demonstrated an impairment of IFN-α and IFN-β production in AEC from pediatric asthmatic patients compared to levels observed in cells from healthy controls [45], [56], [57], [58]. This impairment was also demonstrated in adult cells [59], [60], [61]. Interestingly, similar results have been observed in ex-vivo cultures of PBMC. As early as 2002, Bufe, et al. have shown in an ex-vivo model a defective production of IFN-α in PBMC from pediatric asthmatic patients compared to healthy controls, upon Newcastle disease virus (NDV) infection, an avian respiratory virus belonging to the Paramyxoviridae family and well-known potent inductor of IFN-α [62]. They later extended these observations in whole blood cells cultures from adult asthmatics, upon infection with either NDV of RSV [63]. In the later years, similar results have been shown in pediatric studies [64], [65] but also in adult studies [45], [48], [66]. So far, only a few studies have been conducted upon in vivo inoculation and natural infection. In a cohort of preschool children, IFN-α systemic levels were reduced at the time of RV-induced exacerbation in asthmatics, as compared with healthy controls during an asymptomatic RV infection [30]. In our recently published cohort of preschool asthmatic children with natural virus-induced exacerbation (RV: 73%), the most severe children, who were prone-to-exacerbation, displayed lower plasma concentrations of IFN-β at exacerbation than the others, suggesting a defect of production in response to viruses limited to this sub-population [8]. In another study conducted in older children, we did not observe altered IFN responses in sputum, plasma and cultures of stimulated-PBMC of patients presenting a virus-induced exacerbation, compared with those with a non-virus-induced exacerbation [49]. In adult asthmatics, a significant type I IFN defect was demonstrated in bronchial epithelium and sub-epithelium obtained from bronchial biopsies compared with healthy controls, after experimental infection with RV-A16 [67]. In contrast, Schwantes, et al. described elevated rather than low RNA levels of type I IFN in sputum from adult asthmatic patients, compared to those observed in healthy subjects, early in the course of a virus-induced exacerbation.[68].
Following their discovery in 2003, there has been increasing knowledge regarding the implication of type III IFN implication in anti-viral responses [69]. Ex-vivo studies conducted in AEC from asthmatic subjects have suggested a parallel impairment of IFN-λ1 and IFN-λ2/3 production upon RV infection in pediatric patients [45], [56], [57], and this impairment seemed to persist in adult patients [46], [59]. In vivo, this defective response was not observed at the time of asthma exacerbation in sputum and/or PBMC from atopic asthmatic children [30], [49], [70], nor in adults [68], [71], suggesting that there are compensatory mechanisms or other sources of IFN, supplementing the defect observed in resident cells.
A few studies have explored the potential pathways leading to a defect of IFN production in asthmatic patients. Downregulation of IRF3 or IRF7, following virus infection in asthmatic cells, has been suggested [57], [59]. Gielen, et al. have also demonstrated a role of the molecule suppressor of cytokine signaling 1 (SOCS1), exercising a negative-feedback on IFN production following viral infection in children with STRA [57].
Collectively, these studies suggest a potential impairment of anti-viral IFN response, in particular type I IFN, during asthma attack. Rather than explained by immaturity in the first years of life, this defective response could also be limited to a sub-phenotype of patients who are prone-to-exacerbation, from early childhood to adulthood. The effect of age, the kinetics of the IFN response following viral infection and the impact of viral load in the airways remain to be addressed.
2.3. Cytokines and epithelial-derived alarmins (IL-25, IL-33 and TSLP) response against viral infection
Airway epithelial cells display immunoregulatory properties by producing pro-inflammatory cytokines, including alarmins interleukin (IL)-25, IL-33 and thymic stromal lymphopeietin (TSLP), released upon viral infection, and implicated in the recruitment of macrophages, DC, T cells and granulocytes, such as eosinophils and neutrophils. This will promote the adaptive immune response through DC maturation and migration to draining lymph nodes associated with the airways. Dendritic cells will thus induce naïve T cell differentiation into antigen-specific T cells. This will drive the adaptive immune response within the airways to clear the virus out. The alarmins also contribute to a biased T helper (Th)2 immune response by activating type 2 innate lymphoid cells (ILC2) and polarizing DC in order to promote T cells differentiation (Fig. 1). This specific cytokine environment in asthmatic patients favor naïve T cells polarization into Th2 cells, producing IL-4, IL-5, IL-13, as opposed to Th1 cells, producing IFN-γ and TNF-β [37], [72]. These cytokines are responsible for the type 2 inflammation and induce the pathophysiological features of asthma, including eosinophil mobilization, mucus hypersecretion, smooth muscle hyperplasia and airflow obstruction [3], [73]. Under the influence of IL-5, eosinophils will enter the airways and perpetuate type 2 inflammation [49], [74]. They will also contribute to resolution of immune responses, including tissue repair [75]. Other cytokines, such as type 17 cytokines: IL-17, IL-21, IL-22, can also promote mucus hypersecretion and production of cytokines and chemokines by AEC. They will favor the recruitment of neutrophils, through the induction of C-X-C chemokines, conducting to a state of chronic inflammation [67]. In the longer term, IL-17 may contribute to the development of airway remodeling during asthma by enhancing the production of profibrotic cytokines, proangiogenic factors, proteases and collagen [76], [77].
In children, the influence of respiratory viruses and innate immune mechanisms on the adaptive immune response development may be crucial. First, some respiratory viruses may induce the release of alarmins, and thus favor the development of Th2 inflammation. A recently published study showed that children with RV-positive-bronchiolitis displayed increased IL-4/IFN-γ ratio in nasopharyngeal aspirates [78]. Upon RSV infection, Lee, et al. observed a higher production of TSLP by AEC from asthmatic children compared to healthy controls [79]. Hence, some respiratory viruses may induce Th2 inflammation in the airways of children and this may be increased in an asthmatic environment. In a cohort of preschool asthmatic children, ILC markers, such as soluble ST2, were induced in vivo following RV infection in preschool asthmatic children, and were correlated with nasal levels of IL-33 [80]. In our study of school-age children, at exacerbation, airway inflammation in infected patients was characterized by significantly higher IL-5 concentration and eosinophil count than in non-infected patients [49]. This may not be specific to childhood, as Jackson, et al. have also demonstrated an induction of IL-4, IL-5 and IL-13 in BAL following RV inoculation in asthmatic subjects, associated with exacerbation severity [81]. Additional in vitro experiments showed that IL-33 directly induced Th2 cytokine production by T cells and ILC2. However, this RV-induced Th2 inflammation could be limited to an asthmatic environment in adults, as suggested by an in vitro study reproducing the induction of IL-4 and IL-13 by asthmatic PBMC after co-exposition with RV and IL-33, but showing no effect on PBMC from healthy donors [82]. In addition, exposure to respiratory viruses has also been associated with an increase in Th17 cytokines, as demonstrated in co-cultures of RSV-infected AEC with T cells [83].
Interferon production is also closely linked to Th2 and inflammatory cytokines. In asthmatic preschool children prone to exacerbation, we observed simultaneously lower production of Th1 cytokines (IFN-γ), Th2 cytokines (IL-5), pro-inflammatory cytokines (TNF-α), regulatory cytokines (IL-10), and type 1 IFN (IFN-β) during virus-induced exacerbation in a subgroup of preschool children with characteristics of severe asthma and a history of frequent exacerbations [8]. This association seems to persist in adults, as suggested by Parsons, et al. reporting a parallel decrease of type III IFNs and of IL-6, (C-X-C motif) ligand (CXCL)8 and CXCL10 in adult asthmatic AEC inoculated with RV [46]. In a murine model of allergic asthma, IRF3 controlled the parallel evolution of IFN and Th2 cytokines during acute episodes induced by allergen exposure [84]. Some adult studies have suggested bi-directional influences during viral infection. Upon RV infection, pre-treatment with Th2 cytokines in cultures of AEC may impair TLR3 and IRF3 signaling pathway [59], or type 1 and 3 IFN expression [57]. Lower production of type I IFN, displayed by some children, could also reduce the apoptosis of neighbored-infected cells and increase viral loads, consequently amplifying airway inflammation, inflammatory cytokine production and thus, asthma attack severity [58], [60].
Altogether, these studies confirm close interactions between innate and adaptive immune mechanisms against viruses in an asthmatic environment. Modulating airway inflammation, particularly Th2 inflammation, could therefore directly improve anti-viral responses during asthma attacks in some children.
2.4. Viral behavior during asthma attack
Although the impairment of anti-viral responses plays a key role in asthma attack, characteristics of the microorganisms are also important. First, pathogenicity of RV strongly differs according to subtypes, probably because of the implication of different cellular targets. Indeed, infection with RV-C strains, which use cadherin-related family member 3 (CDHR3) as a receptor, may be associated with higher clinical severity [10], [31], [85], whereas B serotypes seem to be responsible for milder symptoms. Interestingly, single nucleotide polymorphism in the CDHR3 gene, which influences the efficiency of RV-C to infect its target cells, has been associated with childhood asthma susceptibility [86]. Moreover, different viral strains could have different replication rates and thus induce higher viral loads [87]. Rhinovirus C strains could also modulate the airways inflammation, and induce higher levels of Th2 and/or Th17 cytokines, as shown in preschool children with RV-C-induced wheeze [88]. Second, systemic viremia has been observed in children infected with respiratory viruses, such as RV-C, and young age was found to be a risk factor associated with viremia [89]. This state could induce an enhanced inflammation in children by interaction of viruses with systemic immune cells. Finally, viral-coinfection has been frequently observed at exacerbation in asthmatic children, particularly in the preschool years [30], [31]. The combined effects of several viruses within the airways may modulate both the innate and adaptive responses.
These data demonstrate that, in susceptible children, the nature of respiratory viruses, their spreading, as well as their interactions with the host cells may directly impact the inflammation and the severity of asthma exacerbations.
2.5. Emerging role of the airways virome
The role of viral carriage and/or asymptomatic infection at steady state, i.e. outside an exacerbation, in asthmatic patients has also been hypothesized. Hence, pediatric studies have shown a frequent virus shedding in children, with RV positive samples observed in 19–29% of unselected asthmatic children and in 15–23% of healthy children [10], [28], [90], [91]. In the previously-cited cohort of preschool children prone to exacerbation, we observed even higher rates, with 67% of positive PCR (51% for RV) at steady state [8]. In 78 school-age children, we also observed that 10% of children had positive RV sampling at exacerbation and steady state, and that RV serotypes were systematically different at both time points, pointing toward reinfection rather than persistence [10]. Consistent with these observations, Bergauer, et al. also found 54% of RV at steady state in preschool children with asthma, vs 45% in healthy controls [30]. Interestingly, RV carriage in both asthmatics and healthy controls was found to be associated with higher levels of type III IFN at baseline than in subjects without virus. This increase in baseline inflammation seems to persist in adulthood. Hence, in sputum cells from 57 asthmatic adults, da Silva, et al. have shown higher baseline levels of IFN and anti-viral molecules compared with healthy subjects [92]. In all, one could hypothesize that RV persistence in asthmatic patients could induce a pro-inflammatory state and/or a desensitization of PRR, which could reduce the response against pathogens within the airways upon new infection.
Alongside RV, other respiratory viruses, could also take part in these processes. Metagenomic analyses have made it possible to identify hundreds of viral species within the airways, constituting the respiratory tract virome [93]. In non-asthmatic children, Wang, et al. have shown multiple common epidemic respiratory viruses in children with severe acute respiratory infection, whereas, the virome was less diverse and mainly dominated by the Anelloviridae family in healthy children [94]. Anelloviruses are major components of the virome and display immunomodulatory properties on both innate and adaptive immunity [95]. Although their presence has been associated with an impaired lung function in asthmatic children [96], to our knowledge, no study has evaluated their association with asthma attacks. Thus, the contribution of these viruses and their interactions with both pathogenic infectious agents, as well as commensal bacteria from the pulmonary microbiota on chronic inflammation and the onset of acute asthma remain to be determined.
3. The emerging role of pathogenic bacteria and microbiota in asthma
Increasing evidence indicates that the respiratory microbiota, including bacterial and viral microorganisms has an important role in respiratory health and disease [97], [98]. In addition, recent data underline the role of bacterial infections in the development and progression of asthma, as summarized in Table 2 .
Table 2.
Major human studies (pediatric and adult population) assessing the impact of pathogenic bacteria and microbiota during the development, the attack and the natural history of pediatric asthma.
| Compartment | Study | Design and age-group | Main results | |
|---|---|---|---|---|
| Asthma development | Hypopharynx | Bisgaard H, et al. 2007[32] | Hypopharyngeal samples were cultured from 321 neonates at 1 month of age | 21% of children were colonized with S. pneumoniae, M. catarrhalis, H. influenzae, or a combination of these organisms. Colonization with one or more of these organisms, but not colonization with S. aureus, was significantly associated with persistent wheeze. Neonates colonized in the hypopharyngeal region with one or a combination of these organisms, were at increased risk for recurrent wheeze and asthma early in life. |
| Gut microbiota |
Arrieta MC, et al. 2015 [100] |
319 children enrolled in the Canadian Healthy Infant Longitudinal Development (CHILD) Study | Children at risk of asthma exhibited transient gut microbial dysbiosis during the first 100 days of life Relative abundance of the bacterial genera Lachnospira, Veillonella, Faecalibacterium, and Rothia was significantly decreased in children at risk of asthma. This reduction in bacterial taxa was accompanied by reduced levels of fecal acetate. Inoculation of germ-free mice with these four bacterial taxa ameliorated airway inflammation in their adult progeny. |
|
| Lung | Loewen K, et al. 2015[102] | 213, 661 mother–child dyads with a median follow-up time of 9.3 years from birth Maternal antibiotic use was determined from records of oral antibiotic prescriptions |
36.8% of children were exposed prenatally to antibiotics and 10.1% developed asthma. Prenatal antibiotic exposure was associated with an increased risk of asthma. Maternal antibiotic use during 9 months before pregnancy and 9 months postpartum were similarly associated with asthma. |
|
| Hoskin-Parr L, et al. 2013[103] | 4,952 children from the Avon Longitudinal Study of Parents and Children (ALSPAC). Child antibiotic use and asthma, eczema and hay fever symptoms were reported | Children reported to have taken antibiotics during infancy (0–2 yr) were more likely to have asthma. The risk increased with greater numbers of antibiotic courses. The number of courses was also associated with a higher risk of eczema and hay fever but not atopy. |
||
| Gut microbiota | Patrick DM, et al. 2020[104] | 2,644 children from the Canadian Healthy Infant Longitudinal Development (CHILD) prospective birth cohort and among them, 917 were analyzed for fecal microbiota | Reduction in asthma incidence over the study period was associated with decreasing antibiotic use in the first year of life.Asthma diagnosis in childhood was associated with infant antibiotic use (adjusted odds ratio [aOR] 2.15) , with a significant dose–response; α-diversity of the gut microbiota was associated with a 32% reduced risk of asthma at age 5 years. |
|
| Acute asthma | Nasopharynx | Mansbach JM, et al. 2016[106] | The MARC-35 prospective cohort study of 1,016 infants (age less than 1 year) hospitalized with bronchiolitis and followed for the development of recurrent wheezing | RSV infection was associated with high abundance of Firmicutes and Streptococcus and a low abundance of Proteobacteria and the genera Haemophilus and Moraxella. RV infection was associated with low Streptococcus and high Haemophilus and Moraxella. RSV/RV coinfections had intermediate abundances. |
| Nasopharynx | De Steenhuijsen Piters WA, et al. 2016[105] | 106 children less than 2 years of age with RSV infection/26 asymptomatic healthy control | 5 nasopharyngeal microbiota clusters were identified, characterized by high levels of either Haemophilus influenzae, Streptococcus, Corynebacterium, Moraxella, or Staphylococcus aureus. RSV hospitalization were positively associated with H. influenzae and Streptococcus RSV-induced expression of IFN-related genes is independent of the microbiota cluster |
|
| Nasopharynx | Kloepfer KM, et al. 2017[107] | 17 adult subjects collected nasal mucus samples on a weekly basis for 5 consecutive weeks | Asymptomatic RV infections were associated with a significant increase in the abundance of Dolosigranulum and Corynebacterium.RV infection preceded the increased odds of detection of Streptococcus and Moraxella by 1 week. | |
| Blood | Giuffrida LM, et al. 2017[108] | 14 asthmatic and 29 non-asthmatic adult patients with acute respiratory infection 10 healthy individuals |
Higher blood concentrations of CCL2 and CCL5 in infected asthmatic patients than in non-asthmatic patients. Both asthmatic and non-asthmatics with pneumonia or bronchitis had higher concentrations of blood cytokines. | |
| Pharynx | Kama Y, et al. 2020[109] | Pharyngeal samples of outpatients and/or in patients children with acute exacerbations of asthma (n = 111) (median age: 2.8/2.6, respectively) | The 3 major bacterial pathogens were Streptococcus pneumoniae (29.7%), Moraxella catarrhalis (11.7%), and Haemophilus influenzae (10.8%). Patients with S. pneumoniae colonization had significantly shorter wheezing episodes and reduced lung inflammation (including lower level of TNF-α) |
|
| Asthma natural history | Nasopharynx | Teo SM, et al. 2018[110] | 244 infants with acute respiratory tract illnesses (ARIs) followed through their first five years of life | Dominance with Moraxella, Haemophilus and Streptococcus cluster were positively associated with acute respiratory tract illness. This change frequently preceded the detection of viral pathogens and acute symptoms. These changes were associated with ensuing development of persistent wheeze in children developing early allergic sensitization |
| Nasal microbiota | Zhou Y, et al. 2019[113] | A 1 year longitudinal study in school-age children with mild-moderate persistent asthma treated with daily ICS (mean age 8.0 ± 1.8 years) | Children with nasal microbiota dominated by the commensal Corynebacterium / Dolosigranulum cluster at steady state experienced the lowest rates of exacerbation and disease progression. A switch towards the Moraxella- cluster was associated with highest risk of severe asthma exacerbation. |
|
| Lung | Mansbach JM, et al. 2020[111] | 842 infants hospitalized for bronchiolitis with a follow-up at 3 years | Increased abundance of Moraxella or Streptococcus species 3 weeks after day 1 of hospitalization was associated with an increased risk of recurrent wheezing. Increased Streptococcus species abundance the summer after hospitalization was also associated with a greater risk of recurrent wheezing |
|
| Oropharynx | Cuthbertson L, et al. 2019[112] | Oropharyngeal swabs were collected from 109 children hospitalized for acute wheezing/75 non-wheezing controls. | No significant difference in bacterial diversity between wheezers and healthy controls In wheezers, attendance at kindergarten or preschool was, associated with increased bacterial diversity.in contrast with rhinovirus (RV) infection |
|
| Nasopharynx | McCauley K. et al. 2019[35] | 312 school-age asthmatic patients enrolled in a trial of omalizumab, nasal secretion samples collected after randomization | Nasal microbiotas dominated by Moraxella species were associated with increased exacerbation risk and eosinophil activation. Staphylococcus or Corynebacterium species–dominated microbiotas were associated with reduced respiratory illness and exacerbation events. Streptococcus species–dominated microbiota increased the risk of RV. |
|
| Sputum | Abdel-Aziz MI. et al. 2020[114] | Sputum samples were collected in 100 severe asthmatics of the U-BIOPRED adult patients cohort at baseline and after 12–18 months of follow-up | Two microbiome-driven clusters were identified, characterized by asthma onset, smoking status, treatment, lung spirometry results, percentage of neutrophils and macrophages in sputum. Patients of the most severe cluster displayed a commensal-deficient bacterial profile which was associated with worse asthma outcomes. Longitudinal clusters revealed high relative stability after 12–18 months in the severe asthmatics. |
|
| Lung (BAL) | Robinson PFM, et al. 2019[34] |
Children: 35 wheezers categorized as MTW or EVW BAL obtained at steady state |
There was no relationship between lower airway inflammation or infection and clinical preschool wheeze phenotypes. 2 groups identified: 1) a Moraxella species dysbiotic microbiota cluster that associated with airway neutrophilia and a mixed microbiota; 2) a mixed microbiota cluster with a macrophage- and lymphocyte-predominant inflammatory profile |
|
BAL: Broncho-alveolar lavage; EVW: Episodic Viral Wheeze, MTW: Multiple trigger Wheeze, RSV: Respiratory syncytial virus; RV: Rhinovirus.
During the first weeks of life, the respiratory tract begins to develop a niche-specific community pattern, where Staphylococcus aureus and Corynebacterium replace the originally colonizing bacteria and become the dominant microorganisms [98]. The most dramatic changes occur during the first 2 months. In the following six months, the respiratory microbiota continues to mature; relative abundance of S. aureus declines, and an increase in Moraxella, Streptococcus, Haemophilus, Dolosigranulum, Alloiococcus, and Prevotella sp. is observed. This period is crucial and alterations of the microbiota have been implicated in the development and the manifestations of chronic lung diseases.
3.1. Bacteria and asthma development
Using bacterial culture techniques, it has been demonstrated that neonates born to asthmatic mothers with upper airway colonization with S. pneumoniae, M. catarrhalis, and/or H. influenzae were at higher risk of presenting asthma at the age of 5 years [32]. This was associated with an aberrant immune response, with increased IL-5 and IL-13 synthesis in PBMC obtained at the age of 6 months [99]. Interestingly, evaluation of the gut microbiota in infants included in the Canadian (CHILD) study showed that infants at risk of asthma exhibited transient alterations of the microbiota, known as dysbiosis, in neonates [100]. More specifically, the relative abundance of the commensal bacterial genera Lachnospira, Veillonella, Faecalibacterium, and Rothia was significantly decreased in children at risk of asthma. Furthermore, antibiotic-induced gut dysbiosis has been shown to facilitate the development of allergic asthma in experimental murine models and clinical studies [101]. In a population-based cohort, it has been reported that pre- and post-natal antibiotic exposure was associated with a higher asthma risk [102]. No significant difference in bacterial diversity was observed between samples from those with wheeze and healthy controls. Age and attendance at day care or kindergarten were important factors in driving bacterial diversity whereas wheeze and viral infection were not found to be significantly associated to the bacterial communities. In the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, children for whom antibiotics course was reported before the age of 2 years were more likely to have asthma at age 7, although microbiota was not characterized [103]. Similarly, in British Columbia (Canada), “controlled” antibiotic use during infancy was associated with a reduction in the incidence of pediatric asthma, and was related with the preservation of gut microbial communities [104]. Altogether, these data confirm the need to preserve the microbiota in early infancy in order to limit the development of asthma.
3.2. Relationship between bacteria and asthma attack
Bacterial infections potentially contribute to the severity of symptomatic respiratory viral infections and asthma attacks in children. Indeed, there are bidirectional interactions between viruses and airway bacteria that appear to modulate the severity of illness and the likelihood of asthma attack [20]. Pediatric studies have shown that upper respiratory tract colonization with Streptococcus, Haemophilus, and/or Staphylococcus genus during RSV and RV infection increases the risk of hospitalization independently of age and of the presence of asthma [105], [106]. In our previously-cited cohort of preschool children, asthma attacks were frequently associated with viral infection (94%, mostly RV). Pathogenic bacteria were isolated in induced sputum in 56%, mainly H. influenza, M. catharralis or S. pneumoniae [21]. In adult asthmatics, asymptomatic RV infection was associated with increased abundance of Dolosigranulum and Corynebacterium, whereas symptomatic RV infection seemed to be related with a higher frequency of Moraxella [107]. In another adult study, co-infection with virus and bacteria induced higher levels of cytokine/chemokine than bacterial infection [108]. Interestingly, circulating CCL2 and CCL5 concentrations were increased in infected asthmatic patients compared with non-asthmatic patients. Streptococcus species may also influence the course of asthma exacerbation, as shown by Kama, et al. in preschool children [109]. Indeed, patients with S. pneumoniae colonization in the pharyngeal microbiota had significantly shorter wheezing episodes and reduced lung inflammation (including lower levels of TNF-α).
In summary, these data suggest an ability for bacteria to promote asthma attacks and modulate the inflammation during childhood. Targeting bacterial infections during asthma attacks could thus be an alternative strategy in some children.
3.3. Dysbiosis and impact on asthma natural history
Early-life dysbiosis of the microbiota, in particular lung microbiota, may have long-term consequences on asthma natural history. During viral infection in children less than two years old, detection of Moraxella was found to be associated with current wheeze at 5 years old and presence of Streptococcus, Moraxella, or Haemophilus was associated with a high risk of asthma development [110]. Mansbach, et al. showed in a joint modeling analysis adjusting for 16 covariates, including viral trigger, that a higher relative abundance of Moraxella or Streptococcus species 3 weeks after day 1 of hospitalization for severe bronchiolitis was associated with an increased risk of recurrent wheezing [111]. In contrast, no significant difference in microbiota composition was observed between oro-pharyngeal microbiota from children with acute wheezing (0–14 years old) and healthy controls [112]. In this study, the large range of patient ages and day care attendance were important confounding factors, since the microbiota evolves throughout childhood.
In school-age asthmatic children, Zhou, et al. assessed by longitudinal measurements the relationship between nasal airway microbiota and either loss of asthma control, or risk of severe exacerbations [113]. Whereas the commensal Corynebacterium/Dolosigranulum cluster characterized the patients with the lowest risk of disease progression, a switch towards the Moraxella cluster was associated with the highest risk of severe asthma exacerbations. Similarly, in another cohort of school-age asthmatic children, nasal microbiota dominated by Moraxella species was associated with increased exacerbation risk, and eosinophil activation at exacerbation [35]. Staphylococcus or Corynebacterium species–dominated microbiota were linked with reduced respiratory illness and exacerbation event rates, whereas Streptococcus species-dominated microbiota increased the risk of RV infection. Interestingly, using unbiased airway microbiome-driven clustering, Abdel-Aziz, et al. recently described in adult asthmatics two distinct robust severe asthma phenotypes that were associated with airway neutrophilia, and not related to the presence of Moraxella species [114]. Robinson, et al. assessed the lower airway microbiota by performing BAL at steady state in severe preschool wheezers [34]. They reported two profiles: a Moraxella species “dysbiotic” cluster, associated with airway neutrophilia and a “mixed microbiota” cluster associated with macrophage- and lymphocyte-predominant airway inflammation, thus suggesting that bacteria might influence the characteristics of lung inflammation.
Altogether, these data show that airway dysbiosis in early life might contribute to recurrent wheeze and asthma, and to the severity of the disease. Among bacterial species, the early presence of M. Catharralis and H. influenzae during childhood seems to be a marker of evolution toward a severe disease, through the modulation of the inflammatory reaction. In contrast, colonization with S. pneumoniae might have some anti-inflammatory effects. However, the role of bacteria is often exacerbated or modulated by intrinsic (atopy, inflammation characteristics) or extrinsic factors, such as viral infections and antibiotics.
4. Consequences on asthma phenotypes and endotypes
It is now clear that phenotyping and even more so, endotyping asthmatic patients is an essential step to understand the diversity of immune responses at the time of an asthma attack. We will review the commonly described endotypes of childhood asthma, successively based on airway leukocyte infiltrate, profiles of allergic sensitization and nature of asthma trajectories.
Among airway inflammatory phenotypes, neutrophilic asthma appears to be a distinct endotype, associated with asthma severity in adults, and possibly involved in corticosteroid insensitivity [76]. In children, several studies have shown that association of airway neutrophilia with severity is inconstant [115], [116], [117], [118], and may not persist throughout the course of the disease [116], [119]. However, neutrophils present in the airways of severe asthmatic children may have enhanced survival and proinflammatory functions that could increase baseline inflammation [120]. As previously stated, airway neutrophilia has also been associated with a different IFN response, at baseline and at the time of asthma attack, both in adults [66], [92] and pediatric studies [49]. This could have an impact on lung infections. Thus, we have shown that a subgroup of school-aged children with positive viral PCR both at baseline and exacerbation displayed airway neutrophilia [49]. In severe adult asthmatics, airway neutrophilia has also been associated with lesser microbiota diversity in BAL [114], [121]. Finally, experimental RV infection in adult asthmatic subjects directly induces bronchial mucosal neutrophilia [122]. Therefore, the neutrophilic endotype may be characterized by an alteration of airway microbiota and association with viral infections.
Airway eosinophilia is highly frequent in asthmatic children, observed in severe and non-severe asthma, often but not always associated with response to ICS, thus highlighting the diversity of eosinophilic profiles in pediatric asthma [116], [118], [123]. Mouse models and adult studies have suggested a role of IL-5 induced airway eosinophilia as a positive regulator of RV receptor intercellular adhesion molecule (ICAM)-1 on AEC, and a negative regulator of PRR and antiviral responses [124], [125]. However, in children, it remains unclear whether airway eosinophils are involved in the pathophysiology of severe asthma, and in the propensity to develop acute asthma attacks [126]. In addition, peripheral blood eosinophilia, currently used as the basis for selection of eosinophil-targeted biologics, may not be representative of airway eosinophilia in children [127]. Most importantly, it has been reported that eosinophilia is not necessarily associated with higher levels of Th2 cytokines [118], and other cells such as alarmin-stimulated ILC2 may have a greater biological importance in the pathophysiology of asthma attacks [128]. Eosinophils can also have beneficial effects in childhood asthma, as they display regulatory functions and contribute to lung repair and mucosal homeostasis [75]. Peripheral blood eosinophilia has recently been identified has a biomarker predicting the decrease of severity throughout the adolescent years [16]. Finally, it remains to be determined whether eosinophil-targeted biologics, such as anti-IL-5 mepolizumab, are efficient in reducing asthma attacks in children [129]. Overall, as opposed to adults, airway eosinophilia does not appear as the preferential biomarker to classify pediatric patients nor to assess their risk of acute asthma attacks.
Rather than through the recruitment of eosinophils, type 2 inflammation may play a key role in some children’s susceptibility to develop acute asthma attacks through IgE sensitization. Hence, atopy, defined as allergic sensitization, is a key feature associated with risk of asthma attacks in childhood [7], [8], [14]. As shown by latent-class analyses, atopy is also one of the main factors that may account for the heterogeneity of the disease, especially in the preschool years [130], [131] and could drive response to treatment [3]. Pre-existing atopy may play a role in the modulation of IFN response upon viral infection in children, although studies have shown conflicting results [59], [61], [65]. Durrani, et al. have suggested a direct implication of the high-affinity IgE receptor (FcεRI) in this defect, as its cross-linking on pDC from asthmatic allergic patients is associated with reduced IFN secretion upon RV infection [65]. Following virus-induced exacerbation in atopic children, upregulation of FcεRI receptor on monocytes and DC was observed, associated with a concomitant upregulation of pro-inflammatory cytokines, including IL-4 and IL-13 [132], suggesting that respiratory viral infection in atopic children may initiate an atopy-dependent cascade that amplifies virus-induced airway inflammation. This could initiate long-term remodeling and inflammation. Hence, IgE sensitization, especially if occurring early in life and multiple, has been identified as the main factor associated with long-term asthma risk (recurrence of exacerbations, persistence of asthma, impaired lung function) in neonatal cohorts, including MAAS (Great Britain), MAS (Germany), PARIS (France) and PASTURE (Europe) [133], [134], [135], [136].
Describing asthma trajectories in neonatal longitudinal cohorts may precisely allow identifying novel endotypes. For instance, in a birth cohort of children at high risk of atopy, cord blood cells were tested for their ability to produce IFN after exposure to polyinosinic-polycytidylic acid (Poly I:C), a double stranded RNA that mimics viral PAMP [137]. A group of low producers of type I and III IFN response upon stimulation representing 24% of the patients was identified. They displayed higher risk of lower respiratory tract infection and persistent wheeze at the age of 5, suggesting that these clinical outcomes are partly determined by constitutional mechanisms.
Altogether, beyond airway leukocyte and type 2 inflammation, childhood asthma endotypes seem to be associated with the characteristics of airway microbiota and of the associated immune response. Because these might involve dynamic processes, the study of asthma trajectories throughout childhood is crucial. A better characterization of asthma endotypes will be an essential step in order to achieve a personalized medicine.
5. In the future: machine learning and systemic biological approaches
Digital tools provide an innovative way to study the integrated immune response in asthmatic patients. For instance, Custovic, et al. used machine learning in an in vitro model of RV-A16 infection on PBMC extracted from 11 years old children and described 6 clusters of cytokine response to infection [138]. One cluster, with patients from whom PBMC displayed low IFN responses, high pro-inflammatory cytokines, low Th2 responses and moderate regulatory responses, was associated with early-onset asthma and the highest risk of asthma exacerbations and hospitalizations for lower respiratory tract infection.
Omic technologies, known as genomic, epigenomic, transcriptomic, proteomic, single-cell omics, metabolomics, microbiomics, exposomics, are others fast-developing tools, generating a massive amount of data reflecting the biological state of cell populations at a given time, that will certainly make possible in the future to identify new endotypes and their biomarkers [139], [140]. For instance, with the development of the Human Genome Project, genome-wide association studies have allowed to link childhood asthma with association signals in or near genes involved in innate immune response pathways, such as the TLR1 locus, and the IL-33 locus [141], [142]. Transcriptomics analyses, which aim at investigating all RNA transcripts in a biological sample, will certainly enable the identification of a large quantity of genes modulated in certain conditions. A recent study has described 94 distinct gene-expression modules between virus-induced and non-virus-induced exacerbation in nasal and sputum cells from a large cohort of school-age children [143]. In asthma attacks, this tool may also provide information on the activated pathways linked with inflammatory and anti-microbial response [144], [145]. RNA sequencing in nasal lavage cells from asthmatic children at exacerbation led to identify some genes overexpressed as relevant “nodes” linking genes of the inflammatory response, such as IRF7 [144]. Transcriptomic analyses have also been used to identify genes associated with different phenotypes of asthmatic patients [146]. Finally, metabolomics, the study of the metabolites produced in living systems (both humans and microbiota) represents another promising strategy in the upcoming years to address the role of immuno-metabolism as well as lung and gut microbiota in asthma pathophysiology [147], [148].
To sum up, systemic approaches integrating all biologic pathways are now increasingly used to study the mechanisms leading to susceptibility to acute asthma attacks in children. They may facilitate the identification of novel multimodal biomarkers linked with specific endotypes and therapeutic targets.
6. Therapeutic strategies modulating innate immune defenses during asthma attacks
Conventional maintenance treatment, such as ICS, may be insufficient to prevent attacks in children, especially in severe asthma. Severe therapy-resistant asthma is precisely characterized by repeated severe exacerbations despite high dose daily treatment. As the knowledge about infection-underlying pathophysiological processes grows, new therapeutic approaches that may contribute to either improve innate immune responses or limit the occurrence and consequences of infections during asthma attacks have been recently explored (Fig. 2 ).
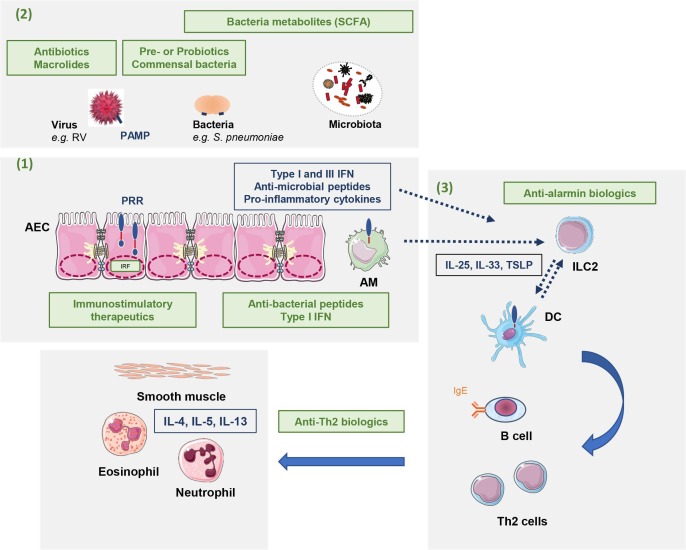
Fig. 2.
Possible therapeutics strategies to limit asthma development/progression and attacks. These strategies (in green) target: (1) Enhancement of the innate immune responses; (2) Anti-infectious therapeutics and strategies to modulate the microbiota; (3) Alarmins and anti-Th2 biologics. AM: Alveolar macrophages; AEC: airway epithelial cells; DC: dendritic cells; IFN: Interferon; IL: Interleukin; ILC2: type 2 innate lymphoid cells; IRF: interferon regulatory factor; PAMP: Pathogen-associated molecular pattern; PRR: pattern recognition receptor; RV: Rhinovirus; SCFA: Short Chain Fatty Acid, TSLP: Thymic stromal lymphopoietin.
6.1. Immunostimulatory therapeutics
As we have shown earlier, asthmatic children may display an impairment of PRR signaling that leads to susceptibility to infection-induced asthma attacks. One of the strategies to enhance their anti-viral responses could involve stimulation of PRR. This strategy has only been tested in pre-clinical and adult studies. Following promising results from pre-clinical studies [149], Silkoff, et al. reported results of a clinical trial assessing the effect of CNTO3157, a TLR3 agonist, upon in vivo inoculation of RV-A16 in adult subjects with mild-to-moderate asthma [150]. The molecule was ineffective in blocking the effect of RV-16 challenge on the onset of asthma exacerbation, without effect on asthma symptoms nor lung function. Additionally, all post-inoculation exacerbations occurred in the CNTO3157 group, and two were severe, thus pointing toward a lack of efficacy and safety of the TLR3 targeting drugs.
Another way of activating PRR and associated signalling pathways could therefore be the administration of PAMP-like adjuvants or particles. Some teams have hypothesized that Poly I:C, a double stranded RNA that activates TLR3 and intracellular receptors, might modulate anti-viral responses. Preclinical studies have shown contrasting results, with promising effect in the context of Influenza A virus infection [52], [151], but concerns regarding its safety. Indeed, Tian, et al. have described, in a mouse model, a prolonged IFN response upon S. pneumonia and S. aureus infection, inducing an impaired bacterial clearance [152]. We have also demonstrated in a murine model of allergic asthma that TLR3 ligands can enhance the Th2 response [153]. Thus, the exact consequences of administering exogenous TLR ligands (including TLR7-8) in humans are still unknown and their potential role in bacterial secondary infections in the lungs need to be addressed.
Another immunomodulatory strategy, particularly appealing in pediatric asthma, could rely on the administration of bacterial lysates [154]. Two main therapies have been described: polyvalent mechanical bacterial lysates (PMBL®) and Broncho-Vaxom OM-85 BV. They contain lysates of 8 pathogenic bacteria including H. influenzae and S. pneumoniae. Both PMBL® and OM-85 administration have proven to be efficient in preventing respiratory tract infections in adult and pediatric patients with chronic bronchitis and/or history of recurrent chronic infections [155], [156], [157], [158], [159]. Interestingly, in a trial conducted in elderly COPD patients, Ricci, et al. observed a reduced number of seroconversion against numerous viral pathogens in patients treated with PMBL®, as well as a better control of the number of all infectious episodes and COPD exacerbations, suggesting that the preventing effect of PMBL® on acute infections was not only on bacterial infections, but also on viral infections [160]. In 152 school-aged children with allergic asthma, Emerick, et al. have described a reduction of exacerbations after 12 weeks of treatment with PMBL®, and no serious adverse event, although no improvement of asthma control scores were achieved [161]. In 75 preschool children with recurrent wheeze, Razi, et al. demonstrated that OM-85 BV reduced the rate and duration of wheezing episodes in the 12 months following the treatment initiation, and reduced the number of acute respiratory tract illnesses [162]. Finally, in toddlers aged 6–18 months, a clinical trial is ongoing to assess the preventive effect of OM-85V on the onset of first wheeze episode related to lower respiratory tract infection during a 3 years observational period [163]. Regarding the mechanisms of action, prevention of acute respiratory infections was not induced by purified LPS or bacterial DNA, suggesting that other PAMP contained in bacterial lysates would activate innate immunity and lead to promotion of type 1 inflammation by DC [164], [165], [166].
Altogether, immunostimulatory therapeutics may represent a promising strategy as additional treatments to prevent asthma attacks but their safety and efficacy need to be properly addressed. Because multiple microorganisms may be involved in acute asthma, it remains also unclear whether they would have an impact on the onset and severity of acute asthma attacks in children.
6.2. Therapeutics enhancing anti-viral responses
Anti-microbial peptides, among which LL-37, are key molecules of the innate immune responses against both viruses and bacteria. Thus, it has been hypothesized that supplementing with LL-37 or inducing its production among AEC could enhance anti-infectious defenses. Sousa, et al. have tested the effect of exogenous cathelicidin on RV-1B replication after inoculation on cultures of AEC [167]. They reported that exogenous LL-37, as well as homologous porcine cathelicidins, either administered prior or after infection, displayed antiviral activity against RV by reducing the metabolic activity of infected cells. However, one preclinical study in a mouse model of asthma showed in vivo a deleterious effect of exogenous cathelicidin-related antimicrobial peptide in airway inflammation and responsiveness [168], and to our knowledge, there is no published study in vivo in humans. Nevertheless, promising results regarding vitamin D supplementation on the release of LL-37 by AEC in in vitro experiments have been published [169], [170], [171]. In clinical studies, there is evidence toward a protective effect of vitamin D supplementation on risk of acute respiratory infections in children [172]. Although there are other effects of vitamin D that might explain its clinical efficacy [80], [173], there is a well-studied biological link between vitamin D and host microbial peptides, via the translocation of vitamin D to the nucleus of AEC, where it activates Vitamin D responsive elements in promoter regions of anti-microbial peptide genes such as LL-37 [52], [171], [174]. Hence, targeting the vitamin D/cathelicidin axis could represent a promising strategy during acute asthma in children.
As studies have shown deficient IFN responses in asthmatic patients, it has been hypothesized that IFN supplementation upon viral infection could enhance anti-viral responses and reduce asthma attacks severity. Interferon therapies (pegylated IFN-α in association with ribavirin) have first been developed in other diseases such as in hepatitis C and human immunodeficiency virus [175], [176]. Ribavirin is a synthetic guanosine nucleoside that impairs the synthesis of viral mRNA [52]. Its clinical utility, upon respiratory virus infection, has been shown in RSV, Adenovirus, RV, or in (SARS)-CoV immunocompromised patients [177], [178], [179], [180]. Since exogenous IFNs reduced RV replication in in vitro cultures of RV-infected AEC from asthmatic patients [181], a trial was initiated in 147 adult asthmatic patients developing symptoms of viral infection (68% RV) to evaluate the efficacy of inhaled IFN-β treatment for 14 days [182]. Overall, it did not improve asthma symptoms, but it enhanced morning peak expiratory flow recovery and reduced the need for additional treatment, i.e. oral corticosteroids or antibiotics. In an exploratory analysis of the subset of more difficult-to-treat patients, IFN supplementation improved asthma control questionnaires. In addition, it boosted anti-viral innate immunity with persistent elevated serum levels of CXCL10, and decreased levels of pro-inflammatory molecules such as CCL4 and CXCL8. In the INEXAS phase 2 trial, designed to assess efficacy of inhaled IFN-β1a for 14 days to prevent asthma exacerbations in adults, McCrae, et al. also reported improvement in the morning peak expiratory flow in the treated arm compared with placebo, but the study stopped early [183], [184]. No study has yet been conducted in pediatric patients.
Collectively, anti-viral therapies could represent potential therapies in the treatment of acute asthma attacks in children. However, the exact timing of administration upon viral infection and onset of acute asthma, and the population of children who might benefit from these strategies remain to be determined.
6.3. Role of anti-microbial therapeutics
Apart from ribavirin, other anti-viral therapies, such as capside-targeting molecules pleconaril, BTA-798 and V-073 have been studied in enteroviruses infections. However, none of these compounds have proven sufficient efficacy and safety in clinical trials [185]. In particular, a phase 2 clinical trial with intranasal pleconaril in children aged 6 years and older, and adults, showed no impact of treatment on the onset of RV-induced cold symptoms or acute asthma exacerbation [186]. Although their efficacy needs to be addressed in clinical trials, DNAzyme therapies could also be promising strategies, as recently suggested by an in vitro study showing good catalytic activity of 2 candidates (Dua-01 and Dua-02) on various RV strains [187].
As described above, bacteria and microbiota may be associated with asthma development and attacks, and antibacterial therapies have emerged as alternative strategies to prevent and/or to treat asthma exacerbation [154], [188], [189]. Treatments aiming to influence the composition of the microbiota are presently tested in different clinical situations and recent data have suggested that these methods might have some interest in asthma. Transplantation of healthy microbiota seems a promising alternative as demonstrated in gut inflammatory diseases [190]. In preclinical study, Arrieta, et al. demonstrated that oral inoculation with four bacterial species previously identified as associated with risk of childhood asthma to germ-free mice improved allergic airway inflammation in their adult offspring [100]. Several studies have also evaluated the benefit of prebiotic and probiotic but their interest during childhood asthma attack has yet to be evaluated [191], [192]. Another option may be to administer bacteria metabolites, which can mimic the effect of normal microbiota. Thus, feeding pregnant mice with short chain fatty acid (SCFA), such as acetate, was associated with modulation of the maternal microbiota and protection from developing allergic airway disease in the offspring [193]. Finally, an increasing number of studies in experimental models of lung infections has demonstrated the potential interest of fecal microbiota transplantation and/or SCFA supplementation, suggesting that they might be efficient in bacteria- and virus-induced exacerbations [194], [195], [196].
The potential modulatory effects of some antibiotics on asthma attacks in children are subject of intense research. First, in terms of prevention, Schwerk, et al. observed a positive impact of long-term antibiotics (mainly amoxicillin and amoxicillin-clavulanic acid) in preschoolers with severe wheeze who had positive bacterial infection on BAL samples, with reduced rates of exacerbations and hospitalizations at 6 months follow-up compared with rates before treatment [197]. Second, immunomodulatory effects of macrolides are well-described [198], and have been evaluated in the treatment of acute asthma in children. Hence, in the Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children (APRIL) study, Bacharier, et al. conducted a pacebo-controlled trial in 607 preschool children with recurrent wheeze and no controller medication, testing the efficacy of azithromycin started prior to the development of lower respiratory tract illness [199]. Macrolide administration reduced the risk of progressing to severe lower respiratory tract illness (i.e requiring a course of oral steroids), without evidence for induction of azithromycin-resistant organisms, nor adverse events. In the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) cohort, children with recurrent asthma-like symptoms, some of whom under maintenance treatment, were randomly assigned to receive either azithromycin or placebo in case of episode of asthma-like symptoms, lasting at least 3 days [200]. Children treated with azithromycin displayed a significant reduction of episode length, and the effect size increased with early administration of treatment. Moreover, Mandhane, et al. conducted a placebo-control trial in preschool children aged 12 to 60 months, presenting to the emergency department with wheeze, to assess the effect of azithromycin [201]. They did not show a reduction of duration of respiratory symptoms in the treated group, neither in the time to the next exacerbation upon 6 months follow-up. Thus, there is still limited evidence on the use of azithromycin as a preventive treatment of acute asthma attacks [202]. In addition, studies have been conducted in adult patients to test the effect of azithromycin as a preventive strategy, with conflicting results [203], [204], [205]. Notably, Gibson, et al. described in a placebo-controlled trial that 48 weeks maintenance treatment with azithromycin decreased the frequency of asthma exacerbations and improved asthma-related quality of life in symptomatic asthma despite current maintenance treatment [204]. In a meta-analysis of the three main studies conducted in adult patients [203], [204], [205], maintenance treatment with azithromycin was associated with a reduced rate of exacerbations in asthma as well as in all subgroups (non-eosinophilic, eosinophilic and severe asthma), and a good tolerance [206]. Examining each exacerbation type separately, patients with eosinophilic asthma reported fewer oral corticosteroid courses, and patients with non-eosinophilic and severe asthma reported fewer antibiotic courses. Additionally, a specific effect of macrolides in neutrophilic asthma had been reported previously by Simpson, et al. in adult subjects with severe refractory asthma, treated with clarithromycin for 8 weeks [207]. Clarithromycin therapy reduced sputum concentrations of IL-8 and neutrophil numbers leading to lower levels of neutrophil elastase and Matrix metallopeptidase (MMP)-9 concentrations versus placebo. Finally, a decrease of inflammatory cytokines, notably IL-1β, IL-6 and IL-33, was observed in vitro in cultures of human AEC from asthmatic patients pretreated with clarithromycin, in a context of RV14 infection, suggesting a direct anti-viral effect [208]. Consistent with this hypothesis, another in vitro study showed an effect of azithromycin to reduce RV replication after inoculation in AEC, associated with an increase in IFN and interferon-stimulated genes mRNA expression and protein production [209].
To sum up, although macrolides and more particularly azithromycin constitute an appealing strategy in acute asthma, further studies are needed to identify the phenotypes of potential responders and the possible adverse events. Since antibiotics have the potential limitation of eliminating commensal bacteria that protect from asthma development, it must be emphasized that their use should only be considered in particular clinical situations [188].
6.4. Potential role of biologics targeting type 2 inflammation in anti-microbial response
We have highlighted the close interactions between underlying airway inflammation, host anti-microbial responses and microorganisms leading to asthma attacks. Therefore, novel therapeutics targeting inflammatory pathways in asthma, such as anti-Th2 inflammation biologics, may have a specific impact on lung infections.
Omalizumab is an anti-IgE monoclonal antibody, targeting the effector mechanisms of Th2 inflammation. After proof of efficacy in pediatric clinical trials, it has been widely prescribed in severe asthmatic atopic children aged older than 6 years, providing more insights on the longer-term evolution [210]. In real-life studies, we have observed higher levels of asthma control after its initiation, and a significant decrease of asthma attacks in children previously prone to exacerbation [211]. Hence, a direct effect of omalizumab on RV-induced exacerbations in children has been suggested [212], [213]. In the Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations (PROSE) study, a placebo-controlled trial conducted among 478 inner-city asthmatic children aged 6–17 years with 1 or more recent exacerbations, Esquivel, et al. observed a decrease of the duration of RV infections, viral shedding, and risk of RV-induced illnesses in omalizumab-treated patients [212]. A recently published adult study including results from two clinical trials has compared the effect of omalizumab on the response to an experimental inoculation with RV-16 in allergic asthmatic adults [214]. Omalizumab-treated patients displayed a reduction of symptoms of lower respiratory tract infection, more pronounced in the four first days following inoculation, suggesting that the protective effect of omalizumab on virus-induced exacerbations occurs in the early stages of infection. Teach, et al. described other results from the PROSE study that compared omalizumab with placebo and omalizumab with an inhaled corticosteroid boost with regard to fall exacerbation rates when initiated 4–6 weeks before return to school [215]. They recorded 86 exacerbations, among which 89% were associated with a respiratory tract virus detection, most commonly RV (81%). More importantly, they observed a significantly lower fall exacerbation rate in the omalizumab group, but no difference between omalizumab and inhaled corticosteroid boost except in the subgroup of patients with exacerbation during the run-in phase. In an ex vivo study of PBMC taken during the intervention phase, the authors observed increased IFN-α responses following RV-A16 inoculation in the omalizumab-treated group, suggesting that the mechanism of Omalizumab protection against exacerbations could involve IFN-α dependent antiviral effect [20]. Another study in children with exacerbation-prone asthma before and during omalizumab treatment described the effect of ex vivo inoculation of RV and Influenza A virus on PBMC and blood pDC, after IgE cross-linking on cell-surface FcεRI [216]. The authors observed that omalizumab increased both virus-induced IFN-α responses in PBMCs and pDC, and subsequently reduced FcεRI expression on pDC. Altogether, the lower asthma exacerbation rates consecutive to omalizumab treatment were related to IFN-α responses and attenuated FcεRI expression on pDC.
Omalizumab remains the most-studied biologic in pediatric asthma but others have been experimented in the past years [217]. Mepolizumab, an anti-IL-5 antibody therapy, has proven to be efficient in reducing the exacerbation rate in adults and is now available in children older than 6 years old with eosinophilic asthma in Europe and the US [218]. As previously stated, its efficacy in reducing exacerbation rates in pediatric clinical trials and real-life studies remain to be determined [129]. However, adult studies suggest it may have a modifying effect on RV-induced inflammatory response. Hence, a placebo-controlled trial was conducted in adult patients with mild asthma, randomized to receive mepolizumab versus placebo, followed by RV-16 inoculation, to study its impact on anti-viral responses [219]. The authors showed that mepolizumab attenuated baseline blood eosinophils numbers and activation status, but did not prevent the activation of remaining eosinophils upon RV infection. However, after infection, it enhanced levels of airway B lymphocytes and AM and modified BAL levels of CCL20 and IL-1RA. Effects of other drugs targeting Th2 cytokines and alarmins, including lebrikizumab (anti IL-13), dupilumab (anti IL-4R), reslizumab (anti-IL-5), benralizumab (anti-IL-5R), tezepelumab (anti-TSLP), or anti-IL-33 monoclonal antibodies are currently studied in pre-clinical and clinical studies [217].
In the next years, new studies are needed regarding these novel treatments, both in allergic and non-allergic asthmatic children, to provide new evidence on their immunological effects and potential disease-modifying properties, and to fully determine the phenotypes/endotypes of good responders. Most importantly, specific studies in the youngest children need to be carried out, with appropriate end-points [129]. This effort will contribute to enter the era of precision medicine in pediatric asthma.
7. Conclusion
In conclusion, asthma attacks are a main feature of pediatric asthma, especially of severe asthma. We have highlighted studies suggesting impairment of the mucosal anti-microbial immune responses during acute asthma and/or at steady state. Interestingly, these alterations seem to inconstantly persist into adulthood. These various altered pathways may reflect different endotypes underlying asthma heterogeneity and consequently, constitute targets for new treatment approaches. Preventing, understanding and efficiently managing acute asthma attacks remain one of the unmet needs in severe childhood asthma, especially in the youngest children. At the era of precision medicine, early interventions targeting mucosal immune responses, lung infections, and microbiota, in early childhood, could have disease-modifying properties and positive impact on the trajectory of childhood asthma.
8. Capsule summary
Characteristics of host-microbiota interactions in asthmatic children favor asthma attacks and may allow to define asthma endotypes. This knowledge offers promising perspectives to treat asthmatic children using precision medicine.
CRediT authorship contribution statement
Stéphanie Lejeune: Conceptualization, Writing - review & editing. Antoine Deschildre: Conceptualization, Writing - review & editing. Olivier Le Rouzic: Writing - review & editing. Ilka Engelmann: Writing - review & editing. Rodrigue Dessein: Writing - review & editing. Muriel Pichavant: Writing - review & editing. Philippe Gosset: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Our studies received grant supports from the “Conseil regional des hauts de France, France“ (Virasthma 1 and Virasthma 2), the Société Française d’Allergologie, and the « Comité National contre les maladies respiratoires, France » (Virasthma 1), the « Fondation du souffle » and Stallergen greer (Virasthma 2). Our research is also supported by INSERM, CNRS and University of Lille, France.
References
- 1.Delmas M.-C., Guignon N., Leynaert B., Moisy M., Marguet C., Fuhrman C. Increase in asthma prevalence among young children in France. Rev. Mal. Respir. 2017;34:525–534. doi: 10.1016/j.rmr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Ebmeier S., Thayabaran D., Braithwaite I., Bénamara C., Weatherall M., Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012) Lancet. 2017;390:935–945. doi: 10.1016/S0140-6736(17)31448-4. [DOI] [PubMed] [Google Scholar]
- 3.Saglani S., Fleming L., Sonnappa S., Bush A. Advances in the aetiology, management, and prevention of acute asthma attacks in children. Lancet Child Adolesc Health. 2019;3:354–364. doi: 10.1016/S2352-4642(19)30025-2. [DOI] [PubMed] [Google Scholar]
- 4.Bloom C.I., Nissen F., Douglas I.J., Smeeth L., Cullinan P., Quint J.K. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax. 2018;73:313–320. doi: 10.1136/thoraxjnl-2017-210650. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma (GINA). 2020 Report: Global strategy for asthma management and prevention. https://ginasthma.org/ (last accessed: April 2020), (n.d.).
- 6.Brand P.L.P., Caudri D., Eber E., Gaillard E.A., Garcia-Marcos L., Hedlin G., Henderson J., Kuehni C.E., Merkus P.J.F.M., Pedersen S., Valiulis A., Wennergren G., Bush A. Classification and pharmacological treatment of preschool wheezing: changes since 2008. Eur. Respir. J. 2014;43:1172–1177. doi: 10.1183/09031936.00199913. [DOI] [PubMed] [Google Scholar]
- 7.Bacharier L.B., Phillips B.R., Bloomberg G.R., Zeiger R.S., Paul I.M., Krawiec M., Guilbert T., Chinchilli V.M., Strunk R.C., Childhood Asthma Research and Education Network, National Heart, Lung, and Blood Institute Severe intermittent wheezing in preschool children: a distinct phenotype. J. Allergy Clin. Immunol. 2007;119:604–610. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 8.Lejeune S., Pichavant M., Engelmann I., Beghin L., Drumez E., Le Rouzic O., Dessein R., Rogeau S., Beke T., Kervoaze G., Delvart C., Ducoin H., Pouessel G., Le Mée A., Boileau S., Roussel J., Bonnel C., Mordacq C., Thumerelle C., Gosset P., Deschildre A. Severe preschool asthmatics have altered cytokine and anti-viral responses during exacerbation. Pediatr. Allergy Immunol. 2020 doi: 10.1111/pai.13268. [DOI] [PubMed] [Google Scholar]
- 9.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L., Symington P., O’Toole S., Myint S.H., Tyrrell D.A. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelmann I., Mordacq C., Gosset P., Tillie-Leblond I., Dewilde A., Thumerelle C., Pouessel G., Deschildre A. Rhinovirus and asthma: reinfection, not persistence. Am. J. Respir. Crit. Care Med. 2013;188:1165–1167. doi: 10.1164/rccm.201303-0585LE. [DOI] [PubMed] [Google Scholar]
- 11.Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J., Adcock I.M., Bateman E.D., Bel E.H., Bleecker E.R., Boulet L.-P., Brightling C., Chanez P., Dahlen S.-E., Djukanovic R., Frey U., Gaga M., Gibson P., Hamid Q., Jajour N.N., Mauad T., Sorkness R.L., Teague W.G. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 12.Hedlin G., Bush A., Lødrup Carlsen K., Wennergren G., De Benedictis F.M., Melén E., Paton J., Wilson N., Carlsen K.-H., Problematic Severe Asthma in Childhood Initiative group Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur. Respir. J. 2010;36:196–201. doi: 10.1183/09031936.00104809. [DOI] [PubMed] [Google Scholar]
- 13.Teague W.G., Phillips B.R., Fahy J.V., Wenzel S.E., Fitzpatrick A.M., Moore W.C., Hastie A.T., Bleecker E.R., Meyers D.A., Peters S.P., Castro M., Coverstone A.M., Bacharier L.B., Ly N.P., Peters M.C., Denlinger L.C., Ramratnam S., Sorkness R.L., Gaston B.M., Erzurum S.C., Comhair S.A.A., Myers R.E., Zein J., DeBoer M.D., Irani A.-M., Israel E., Levy B., Cardet J.C., Phipatanakul W., Gaffin J.M., Holguin F., Fajt M.L., Aujla S.J., Mauger D.T., Jarjour N.N. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J. Allergy Clin. Immunol. Pract. 2018;6:545–554.e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming L., Murray C., Bansal A.T., Hashimoto S., Bisgaard H., Bush A., Frey U., Hedlin G., Singer F., van Aalderen W.M., Vissing N.H., Zolkipli Z., Selby A., Fowler S., Shaw D., Chung K.F., Sousa A.R., Wagers S., Corfield J., Pandis I., Rowe A., Formaggio E., Sterk P.J., Roberts G., U-BIOPRED Study Group The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur. Respir. J. 2015;46:1322–1333. doi: 10.1183/13993003.00780-2015. [DOI] [PubMed] [Google Scholar]
- 15.Denlinger L.C., Heymann P., Lutter R., Gern J.E. Exacerbation-prone asthma. J Allergy Clin Immunol Pract. 2020;8:474–482. doi: 10.1016/j.jaip.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross K.R., Gupta R., DeBoer M.D., Zein J., Phillips B.R., Mauger D.T., Li C., Myers R.E., Phipatanakul W., Fitzpatrick A.M., Ly N.P., Bacharier L.B., Jackson D.J., Celedón J.C., Larkin A., Israel E., Levy B., Fahy J.V., Castro M., Bleecker E.R., Meyers D., Moore W.C., Wenzel S.E., Jarjour N.N., Erzurum S.C., Teague W.G., Gaston B. Severe asthma during childhood and adolescence: a longitudinal study. J. Allergy Clin. Immunol. 2020;145:140–146.e9. doi: 10.1016/j.jaci.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Belgrave D.C.M., Buchan I., Bishop C., Lowe L., Simpson A., Custovic A. Trajectories of lung function during childhood. Am. J. Respir. Crit. Care Med. 2014;189:1101–1109. doi: 10.1164/rccm.201309-1700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beigelman A., Bacharier L.B. Management of preschool children with recurrent wheezing: lessons from the NHLBI’s asthma research networks. J. Allergy Clin. Immunol. 2016;4(1):1–8. doi: 10.1016/j.jaip.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourdin A., Adcock I., Berger P., Bonniaud P., Chanson P., Chenivesse C., de Blic J., Deschildre A., Devillier P., Devouassoux G., Didier A., Garcia G., Magnan A., Martinat Y., Perez T., Roche N., Taillé C., Val P., Chanez P. How can we minimise the use of regular oral corticosteroids in asthma? Eur. Respir. Rev. 2020;29 doi: 10.1183/16000617.0085-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Licari A., Manti S., Castagnoli R., Marseglia A., Foiadelli T., Brambilla I., Marseglia G.L. Immunomodulation in pediatric asthma. Front. Pediatr. 2019;7:289. doi: 10.3389/fped.2019.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jartti T., Gern J.E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T., Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr. Allergy Immunol. 2011;22:350–355. doi: 10.1111/j.1399-3038.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 23.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E., Printz M.C., Lee W.-M., Shult P.A., Reisdorf E., Carlson-Dakes K.T., Salazar L.P., DaSilva D.F., Tisler C.J., Gern J.E., Lemanske R.F. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukkarinen M., Koistinen A., Turunen R., Lehtinen P., Vuorinen T., Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J. Allergy Clin. Immunol. 2017;140:988–995. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergroth E., Aakula M., Korppi M., Remes S., Kivistö J.E., Piedra P.A., Camargo C.A., Jartti T. Post-bronchiolitis use of asthma medication: a prospective 1-year follow-up study. Pediatr. Infect. Dis. J. 2016;35:363–368. doi: 10.1097/INF.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 26.Rubner F.J., Jackson D.J., Evans M.D., Gangnon R.E., Tisler C.J., Pappas T.E., Gern J.E., Lemanske R.F. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J. Allergy Clin. Immunol. 2017;139:501–507. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thumerelle C., Deschildre A., Bouquillon C., Santos C., Sardet A., Scalbert M., Delbecque L., Debray P., Dewilde A., Turck D., Leclerc F. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: a prospective study in the Nord-Pas de Calais region (France) Pediatr. Pulmonol. 2003;35:75–82. doi: 10.1002/ppul.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khetsuriani N., Kazerouni N.N., Erdman D.D., Lu X., Redd S.C., Anderson L.J., Teague W.G. Prevalence of viral respiratory tract infections in children with asthma. J. Allergy Clin. Immunol. 2007;119:314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy J.L., Shaker M., McMeen V., Gern J., Carper H., Murphy D., Lee W.-M., Bochkov Y.A., Vrtis R.F., Platts-Mills T., Patrie J., Borish L., Steinke J.W., Woods W.A., Heymann P.W. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am. J. Respir. Crit. Care Med. 2014;189:532–539. doi: 10.1164/rccm.201310-1767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergauer A., Sopel N., Kroß B., Vuorinen T., Xepapadaki P., Weiss S.T., Blau A., Sharma H., Kraus C., Springel R., Rauh M., Mittler S., Graser A., Zimmermann T., Melichar V.O., Kiefer A., Kowalski M.L., Sobanska A., Jartti T., Lukkarinen H., Papadopoulos N.G., Finotto S. IFN-α/IFN-λ responses to respiratory viruses in paediatric asthma. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.00969-2016. [DOI] [PubMed] [Google Scholar]
- 31.Lejeune S., Engelmann I., Dessein R., Pouessel G., Ducoin H., Delvart C., Mordacq C., Thumerelle C., Pichavant M., Gosset P., Deschildre A. Microbiological status in preschool asthma: the VIRASTHMA 2 study. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.congress-2019.OA4938. [DOI] [Google Scholar]
- 32.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bønnelykke K., Brasholt M., Heltberg A., Vissing N.H., Thorsen S.V., Stage M., Pipper C.B. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 33.Kloepfer K.M., Lee W.M., Pappas T.E., Kang T.J., Vrtis R.F., Evans M.D., Gangnon R.E., Bochkov Y.A., Jackson D.J., Lemanske R.F., Gern J.E. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014;133:1301–1307. doi: 10.1016/j.jaci.2014.02.030. 1307.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson P.F.M., Pattaroni C., Cook J., Gregory L., Alonso A.M., Fleming L.J., Lloyd C.M., Bush A., Marsland B.J., Saglani S. Lower airway microbiota associates with inflammatory phenotype in severe preschool wheeze. J. Allergy Clin. Immunol. 2019;143:1607–1610.e3. doi: 10.1016/j.jaci.2018.12.985. [DOI] [PubMed] [Google Scholar]
- 35.McCauley K., Durack J., Valladares R., Fadrosh D.W., Lin D.L., Calatroni A., LeBeau P.K., Tran H.T., Fujimura K.E., LaMere B., Merana G., Lynch K., Cohen R.T., Pongracic J., Khurana Hershey G.K., Kercsmar C.M., Gill M., Liu A.H., Kim H., Kattan M., Teach S.J., Togias A., Boushey H.A., Gern J.E., Jackson D.J., Lynch S.V., National Institute of Allergy and Infectious Diseases-sponsored Inner-City Asthma Consortium Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J. Allergy Clin. Immunol. 2019;144:1187–1197. doi: 10.1016/j.jaci.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vareille M., Kieninger E., Edwards M.R., Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush A. Cytokines and Chemokines as Biomarkers of Future Asthma. Front. Pediatr. 2019;7:72. doi: 10.3389/fped.2019.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hossain F.M.A., Choi J.Y., Uyangaa E., Park S.O., Eo S.K. The interplay between host immunity and respiratory viral infection in asthma exacerbation. Immune Netw. 2019;19 doi: 10.4110/in.2019.19.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitsett J.A., Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadowaki N., Ho S., Antonenko S., Malefyt R.W., Kastelein R.A., Bazan F., Liu Y.J. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barchet W., Blasius A., Cella M., Colonna M. Plasmacytoid dendritic cells: in search of their niche in immune responses. Immunol. Res. 2005;32:75–83. doi: 10.1385/IR:32:1-3:075. [DOI] [PubMed] [Google Scholar]
- 42.See H., Wark P. Innate immune response to viral infection of the lungs. Paediatr. Respir. Rev. 2008;9:243–250. doi: 10.1016/j.prrv.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duez C., Gosset P., Tonnel A.-B. Dendritic cells and toll-like receptors in allergy and asthma. Eur J Dermatol. 2006;16:12–16. [PubMed] [Google Scholar]
- 44.Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 45.Edwards M.R., Regamey N., Vareille M., Kieninger E., Gupta A., Shoemark A., Saglani S., Sykes A., Macintyre J., Davies J., Bossley C., Bush A., Johnston S.L. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parsons K.S., Hsu A.C., Wark P.A.B. TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin. Exp. Allergy. 44. 2014:91–101. doi: 10.1111/cea.12218. [DOI] [PubMed] [Google Scholar]
- 47.Rupani H., Martinez-Nunez R.T., Dennison P., Lau L.C.K., Jayasekera N., Havelock T., Francisco-Garcia A.S., Grainge C., Howarth P.H., Sanchez-Elsner T. Toll-like receptor 7 is reduced in severe asthma and linked to an altered microRNA profile. Am. J. Respir. Crit. Care Med. 2016;194:26–37. doi: 10.1164/rccm.201502-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sykes A., Edwards M.R., Macintyre J., del Rosario A., Bakhsoliani E., Trujillo-Torralbo M.-B., Kon O.M., Mallia P., McHale M., Johnston S.L. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J. Allergy Clin. Immunol. 2012;129:1506–1514.e6. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Deschildre A., Pichavant M., Engelmann I., Langlois C., Drumez E., Pouessel G., Boileau S., Romero-Cubero D., Decleyre-Badiu I., Dewilde A., Hober D., Néve V., Thumerelle C., Lejeune S., Mordacq C., Gosset P. Virus-triggered exacerbation in allergic asthmatic children: neutrophilic airway inflammation and alteration of virus sensors characterize a subgroup of patients. Respir. Res. 2017;18:191. doi: 10.1186/s12931-017-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lejeune S., Mordacq C., Drumez E., Brisset S., Pouessel G., Pichavant M., Engelmann I., Béghin L., Decleyre-Badiu I., Neve V., Thumerelle C., Gosset P., Deschildre A. Relationship between immune parameters during a severe exacerbation in allergic asthmatic children and asthma outcomes in the following year. Clin. Exp. Allergy. 2020 doi: 10.1111/cea.13570. [DOI] [PubMed] [Google Scholar]
- 51.Jackson D.J., Johnston S.L. The role of viruses in acute exacerbations of asthma. J. Allergy Clin. Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. quiz 1188–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casanova V., Sousa F.H., Stevens C., Barlow P.G. Antiviral therapeutic approaches for human rhinovirus infections. Future Virol. 2018;13:505–518. doi: 10.2217/fvl-2018-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barlow P.G., Findlay E.G., Currie S.M., Davidson D.J. Antiviral potential of cathelicidins. Future Microbiol. 2014;9:55–73. doi: 10.2217/fmb.13.135. [DOI] [PubMed] [Google Scholar]
- 54.Currie S.M., Gwyer Findlay E., McFarlane A.J., Fitch P.M., Böttcher B., Colegrave N., Paras A., Jozwik A., Chiu C., Schwarze J., Davidson D.J. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J. Immunol. 2016;196:2699–2710. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arikoglu T., Kuyucu S., Karaismailoglu E., Batmaz S.B., Balci S. The association of vitamin D, cathelicidin, and vitamin D binding protein with acute asthma attacks in children. Allergy Asthma Proc. 2015;36:51–58. doi: 10.2500/aap.2015.36.3848. [DOI] [PubMed] [Google Scholar]
- 56.Baraldo S., Contoli M., Bazzan E., Turato G., Padovani A., Marku B., Calabrese F., Caramori G., Ballarin A., Snijders D., Barbato A., Saetta M., Papi A. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J. Allergy Clin. Immunol. 2012;130:1307–1314. doi: 10.1016/j.jaci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Gielen V., Sykes A., Zhu J., Chan B., Macintyre J., Regamey N., Kieninger E., Gupta A., Shoemark A., Bossley C., Davies J., Saglani S., Walker P., Nicholson S.E., Dalpke A.H., Kon O.-M., Bush A., Johnston S.L., Edwards M.R. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J. Allergy Clin. Immunol. 2015;136:177–188.e11. doi: 10.1016/j.jaci.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kicic A., Stevens P.T., Sutanto E.N., Kicic-Starcevich E., Ling K.-M., Looi K., Martinovich K.M., Garratt L.W., Iosifidis T., Shaw N.C., Buckley A.G., Rigby P.J., Lannigan F.J., Knight D.A., Stick S.M. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin. Exp. Allergy. 2016;46:1441–1455. doi: 10.1111/cea.12767. [DOI] [PubMed] [Google Scholar]
- 59.Contoli M., Ito K., Padovani A., Poletti D., Marku B., Edwards M.R., Stanciu L.A., Gnesini G., Pastore A., Spanevello A., Morelli P., Johnston S.L., Caramori G., Papi A. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015;70:910–920. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 60.Wark P.A.B., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V., Holgate S.T., Davies D.E. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moskwa S., Piotrowski W., Marczak J., Pawełczyk M., Lewandowska-Polak A., Jarzębska M., Brauncajs M., Głobińska A., Górski P., Papadopoulos N.G., Edwards M.R., Johnston S.L., Kowalski M.L. Innate immune response to viral infections in primary bronchial epithelial cells is modified by the atopic status of asthmatic patients. Allergy Asthma Immunol Res. 2018;10:144–154. doi: 10.4168/aair.2018.10.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bufe A., Gehlhar K., Grage-Griebenow E., Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int. Arch. Allergy Immunol. 2002;127:82–88. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- 63.Gehlhar K., Bilitewski C., Reinitz-Rademacher K., Rohde G., Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin. Exp. Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 64.Iikura K., Katsunuma T., Saika S., Saito S., Ichinohe S., Ida H., Saito H., Matsumoto K. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int. Arch. Allergy Immunol. 2011;155(Suppl 1):27–33. doi: 10.1159/000327262. [DOI] [PubMed] [Google Scholar]
- 65.Durrani S.R., Montville D.J., Pratt A.S., Sahu S., DeVries M.K., Rajamanickam V., Gangnon R.E., Gill M.A., Gern J.E., Lemanske R.F., Jackson D.J. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J. Allergy Clin. Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simpson J.L., Carroll M., Yang I.A., Reynolds P.N., Hodge S., James A.L., Gibson P.G., Upham J.W. Reduced antiviral interferon production in poorly controlled asthma is associated with neutrophilic inflammation and high-dose inhaled corticosteroids. Chest. 2016;149:704–713. doi: 10.1016/j.chest.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 67.Zhu J., Message S.D., Mallia P., Kebadze T., Contoli M., Ward C.K., Barnathan E.S., Mascelli M.A., Kon O.M., Papi A., Stanciu L.A., Edwards M.R., Jeffery P.K., Johnston S.L. Bronchial mucosal IFN-α/β and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J. Allergy Clin. Immunol. 2019;143:114–125.e4. doi: 10.1016/j.jaci.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwantes E.A., Manthei D.M., Denlinger L.C., Evans M.D., Gern J.E., Jarjour N.N., Mathur S.K. Interferon gene expression in sputum cells correlates with the Asthma Index Score during virus-induced exacerbations. Clin. Exp. Allergy. 2014;44:813–821. doi: 10.1111/cea.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K., Langer J.A., Sheikh F., Dickensheets H., Donnelly R.P. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 70.Miller E.K., Hernandez J.Z., Wimmenauer V., Shepherd B.E., Hijano D., Libster R., Serra M.E., Bhat N., Batalle J.P., Mohamed Y., Reynaldi A., Rodriguez A., Otello M., Pisapia N., Bugna J., Bellabarba M., Kraft D., Coviello S., Ferolla F.M., Chen A., London S.J., Siberry G.K., Williams J.V., Polack F.P. A mechanistic role for type III IFN-λ1 in asthma exacerbations mediated by human rhinoviruses. Am. J. Respir. Crit. Care Med. 2012;185:508–516. doi: 10.1164/rccm.201108-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A.B., Bartlett N.W., Kebadze T., Mallia P., Stanciu L.A., Parker H.L., Slater L., Lewis-Antes A., Kon O.M., Holgate S.T., Davies D.E., Kotenko S.V., Papi A., Johnston S.L. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 72.Boonpiyathad T., Sözener Z.C., Satitsuksanoa P., Akdis C.A. Immunologic mechanisms in asthma. Semin. Immunol. 2019;46 doi: 10.1016/j.smim.2019.101333. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell P.D., O’Byrne P.M. Epithelial-derived cytokines in asthma. Chest. 2017;151:1338–1344. doi: 10.1016/j.chest.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 74.Gao H., Ying S., Dai Y. Pathological roles of neutrophil-mediated inflammation in asthma and its potential for therapy as a target. J Immunol Res. 2017;2017:3743048. doi: 10.1155/2017/3743048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Travers J., Rothenberg M.E. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8:464–475. doi: 10.1038/mi.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chesné J., Braza F., Mahay G., Brouard S., Aronica M., Magnan A. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 2014;190:1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 77.Bellini A., Marini M.A., Bianchetti L., Barczyk M., Schmidt M., Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5:140–149. doi: 10.1038/mi.2011.60. [DOI] [PubMed] [Google Scholar]
- 78.Yuan X.-H., Li Y.-M., Shen Y.-Y., Yang J., Jin Y. Clinical and Th1/Th2 immune response features of hospitalized children with human rhinovirus infection. J. Med. Virol. 2020;92:26–33. doi: 10.1002/jmv.25587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee H.-C., Headley M.B., Loo Y.-M., Berlin A., Gale M., Debley J.S., Lukacs N.W., Ziegler S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012;130:1187–1196.e5. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haag P., Sharma H., Rauh M., Zimmermann T., Vuorinen T., Papadopoulos N.G., Weiss S.T., Finotto S. Soluble ST2 regulation by rhinovirus and 25(OH)-vitamin D3 in the blood of asthmatic children. Clin. Exp. Immunol. 2018;193:207–220. doi: 10.1111/cei.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson D.J., Makrinioti H., Rana B.M.J., Shamji B.W.H., Trujillo-Torralbo M.-B., Footitt J., null Jerico Del-Rosario, Telcian A.G., Nikonova A., Zhu J., Aniscenko J., Gogsadze L., Bakhsoliani E., Traub S., Dhariwal J., Porter J., Hunt D., Hunt T., Hunt T., Stanciu L.A., Khaitov M., Bartlett N.W., Edwards M.R., Kon O.M., Mallia P., Papadopoulos N.G., Akdis C.A., Westwick J., Edwards M.J., Cousins D.J., Walton R.P., Johnston S.L. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jurak L.M., Xi Y., Landgraf M., Carroll M.L., Murray L., Upham J.W. Interleukin 33 selectively augments rhinovirus-induced type 2 immune responses in asthmatic but not healthy people. Front. Immunol. 2018;9:1895. doi: 10.3389/fimmu.2018.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin L., Hu C., Feng J., Xia Q. Activation of lymphocytes induced by bronchial epithelial cells with prolonged RSV infection. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0027113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marichal T., Bedoret D., Mesnil C., Pichavant M., Goriely S., Trottein F., Cataldo D., Goldman M., Lekeux P., Bureau F., Desmet C.J. Interferon response factor 3 is essential for house dust mite-induced airway allergy. J. Allergy Clin. Immunol. 2010;126:836–844.e13. doi: 10.1016/j.jaci.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 85.Bizzintino J., Lee W.-M., Laing I.A., Vang F., Pappas T., Zhang G., Martin A.C., Khoo S.-K., Cox D.W., Geelhoed G.C., McMinn P.C., Goldblatt J., Gern J.E., Le Souëf P.N. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 2011;37:1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bochkov Y.A., Watters K., Ashraf S., Griggs T.F., Devries M.K., Jackson D.J., Palmenberg A.C., Gern J.E. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. PNAS. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakagome K., Bochkov Y.A., Ashraf S., Brockman-Schneider R.A., Evans M.D., Pasic T.R., Gern J.E. Effects of rhinovirus species on viral replication and cytokine production. J. Allergy Clin. Immunol. 2014;134:332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sikazwe C.T., Laing I.A., Imrie A., Smith D.W. Nasal cytokine profiles of patients hospitalised with respiratory wheeze associated with rhinovirus C. Viruses. 2019;11 doi: 10.3390/v11111038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu X., Schneider E., Jain S., Bramley A.M., Hymas W., Stockmann C., Ampofo K., Arnold S.R., Williams D.J., Self W.H., Patel A., Chappell J.D., Grijalva C.G., Anderson E.J., Wunderink R.G., McCullers J.A., Edwards K.M., Pavia A.T., Erdman D.D. Rhinovirus viremia in patients hospitalized with community-acquired pneumonia. J. Infect. Dis. 2017;216:1104–1111. doi: 10.1093/infdis/jix455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Principi N., Zampiero A., Gambino M., Scala A., Senatore L., Lelii M., Ascolese B., Pelucchi C., Esposito S. Prospective evaluation of rhinovirus infection in healthy young children. J. Clin. Virol. 2015;66:83–89. doi: 10.1016/j.jcv.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Loeffelholz M.J., Trujillo R., Pyles R.B., Miller A.L., Alvarez-Fernandez P., Pong D.L., Chonmaitree T. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics. 2014;134:1144–1150. doi: 10.1542/peds.2014-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.da Silva J., Hilzendeger C., Moermans C., Schleich F., Henket M., Kebadze T., Mallia P., Edwards M.R., Johnston S.L., Louis R. Raised interferon-β, type 3 interferon and interferon-stimulated genes - evidence of innate immune activation in neutrophilic asthma. Clin. Exp. Allergy. 2017;47:313–323. doi: 10.1111/cea.12809. [DOI] [PubMed] [Google Scholar]
- 93.Wylie K.M. The virome of the human respiratory tract. Clin. Chest Med. 2017;38:11–19. doi: 10.1016/j.ccm.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y., Zhu N., Li Y., Lu R., Wang H., Liu G., Zou X., Xie Z., Tan W. Metagenomic analysis of viral genetic diversity in respiratory samples from children with severe acute respiratory infection in China. Clin. Microbiol. Infect. 2016;22(458):e1–9. doi: 10.1016/j.cmi.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freer G., Maggi F., Pifferi M., Di Cicco M.E., Peroni D.G., Pistello M. The virome and its major component, anellovirus, a convoluted system molding human immune defenses and possibly affecting the development of asthma and respiratory diseases in childhood. Front. Microbiol. 2018;9:686. doi: 10.3389/fmicb.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pifferi M., Maggi F., Andreoli E., Lanini L., Marco E.D., Fornai C., Vatteroni M.L., Pistello M., Ragazzo V., Macchia P., Boner A., Bendinelli M. Associations between nasal torquetenovirus load and spirometric indices in children with asthma. J. Infect. Dis. 2005;192:1141–1148. doi: 10.1086/444389. [DOI] [PubMed] [Google Scholar]
- 97.Dinwiddie D.L., Denson J.L., Kennedy J.L. Role of the airway microbiome in respiratory infections and asthma in children. Pediatr Allergy Immunol Pulmonol. 2018;31:236–240. doi: 10.1089/ped.2018.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stiemsma L.T., Michels K.B. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larsen J.M., Brix S., Thysen A.H., Birch S., Rasmussen M.A., Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J. Allergy Clin. Immunol. 2014;133:1008–1013. doi: 10.1016/j.jaci.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Arrieta M.-C., Stiemsma L.T., Dimitriu P.A., Thorson L., Russell S., Yurist-Doutsch S., Kuzeljevic B., Gold M.J., Britton H.M., Lefebvre D.L., Subbarao P., Mandhane P., Becker A., McNagny K.M., Sears M.R., Kollmann T., CHILD Study Investigators, Mohn W.W., Turvey S.E., Finlay B.B. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 7. 2015 doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 101.Hufnagl K., Pali-Schöll I., Roth-Walter F., Jensen-Jarolim E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020;42:75–93. doi: 10.1007/s00281-019-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Loewen K., Monchka B., Mahmud S.M., ’tJong G., Azad M.B. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur. Respir. J. 2018;52 doi: 10.1183/13993003.02070-2017. [DOI] [PubMed] [Google Scholar]
- 103.Hoskin-Parr L., Teyhan A., Blocker A., Henderson A.J.W. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr. Allergy Immunol. 2013;24:762–771. doi: 10.1111/pai.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patrick D.M., Sbihi H., Dai D.L.Y., Al Mamun A., Rasali D., Rose C., Marra F., Boutin R.C.T., Petersen C., Stiemsma L.T., Winsor G.L., Brinkman F.S.L., Kozyrskyj A.L., Azad M.B., Becker A.B., Mandhane P.J., Moraes T.J., Sears M.R., Subbarao P., Finlay B.B., Turvey S.E. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30052-7. [DOI] [PubMed] [Google Scholar]
- 105.de Steenhuijsen Piters W.A.A., Heinonen S., Hasrat R., Bunsow E., Smith B., Suarez-Arrabal M.-C., Chaussabel D., Cohen D.M., Sanders E.A.M., Ramilo O., Bogaert D., Mejias A. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am. J. Respir. Crit. Care Med. 2016;194:1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mansbach J.M., Hasegawa K., Henke D.M., Ajami N.J., Petrosino J.F., Shaw C.A., Piedra P.A., Sullivan A.F., Espinola J.A., Camargo C.A. Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J. Allergy Clin. Immunol. 2016;137:1909–1913.e4. doi: 10.1016/j.jaci.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kloepfer K.M., Sarsani V.K., Poroyko V., Lee W.M., Pappas T.E., Kang T., Grindle K.A., Bochkov Y.A., Janga S.C., Lemanske R.F., Gern J.E. Community-acquired rhinovirus infection is associated with changes in the airway microbiome. J. Allergy Clin. Immunol. 2017;140:312–315.e8. doi: 10.1016/j.jaci.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giuffrida M.J., Valero N., Mosquera J., Duran A., Arocha F., Chacín B., Espina L.M., Gotera J., Bermudez J., Mavarez A., Alvarez-Mon M. Increased systemic cytokine/chemokine expression in asthmatic and non-asthmatic patients with bacterial, viral or mixed lung infection. Scand. J. Immunol. 2017;85:280–290. doi: 10.1111/sji.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kama Y., Kato M., Yamada Y., Koike T., Suzuki K., Enseki M., Tabata H., Hirai K., Mochizuki H. The suppressive role of Streptococcus pneumoniae colonization in acute exacerbations of childhood bronchial asthma. Int. Arch. Allergy Immunol. 2020;181:191–199. doi: 10.1159/000504541. [DOI] [PubMed] [Google Scholar]
- 110.Teo S.M., Tang H.H.F., Mok D., Judd L.M., Watts S.C., Pham K., Holt B.J., Kusel M., Serralha M., Troy N., Bochkov Y.A., Grindle K., Lemanske R.F., Johnston S.L., Gern J.E., Sly P.D., Holt P.G., Holt K.E., Inouye M. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe. 2018;24:341–352.e5. doi: 10.1016/j.chom.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mansbach J.M., Luna P.N., Shaw C.A., Hasegawa K., Petrosino J.F., Piedra P.A., Sullivan A.F., Espinola J.A., Stewart C.J., Camargo C.A. Increased Moraxella and Streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing. J. Allergy Clin. Immunol. 2020;145:518–527.e8. doi: 10.1016/j.jaci.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cuthbertson L., Oo S.W.C., Cox M.J., Khoo S.-K., Cox D.W., Chidlow G., Franks K., Prastanti F., Borland M.L., Gern J.E., Smith D.W., Bizzintino J.A., Laing I.A., Le Souëf P.N., Moffatt M.F., Cookson W.O.C. Viral respiratory infections and the oropharyngeal bacterial microbiota in acutely wheezing children. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0223990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Y., Jackson D., Bacharier L.B., Mauger D., Boushey H., Castro M., Durack J., Huang Y., Lemanske R.F., Storch G.A., Weinstock G.M., Wylie K., Covar R., Fitzpatrick A.M., Phipatanakul W., Robison R.G., Beigelman A. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat. Commun. 2019;10:5714. doi: 10.1038/s41467-019-13698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abdel-Aziz M.I., Brinkman P., Vijverberg S.J.H., Neerincx A.H., Riley J.H., Bates S., Hashimoto S., Kermani N.Z., Chung K.F., Djukanovic R., Dahlén S.-E., Adcock I.M., Howarth P.H., Sterk P.J., Kraneveld A.D., Maitland-van der Zee A.H. U-BIOPRED Study Group, Sputum microbiome profiles identify severe asthma phenotypes of relative stability at 12–18 months. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 115.Lezmi G., de Blic J. Assessment of airway inflammation and remodeling in children with severe asthma: the next challenge. Pediatr. Pulmonol. 2018;53:1171–1173. doi: 10.1002/ppul.24051. [DOI] [PubMed] [Google Scholar]
- 116.Lezmi G., Deschildre A., Abou Taam R., Fayon M., Blanchon S., Troussier F., Mallinger P., Mahut B., Gosset P., de Blic J. Remodelling and inflammation in preschoolers with severe recurrent wheeze and asthma outcome at school age. Clin. Exp. Allergy. 2018;48:806–813. doi: 10.1111/cea.13143. [DOI] [PubMed] [Google Scholar]
- 117.Ray A., Kolls J.K. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017;38:942–954. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bossley C.J., Fleming L., Gupta A., Regamey N., Frith J., Oates T., Tsartsali L., Lloyd C.M., Bush A., Saglani S. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J. Allergy Clin. Immunol. 2012;129:974–982.e13. doi: 10.1016/j.jaci.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fleming L., Tsartsali L., Wilson N., Regamey N., Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012;67:675–681. doi: 10.1136/thoraxjnl-2011-201064. [DOI] [PubMed] [Google Scholar]
- 120.Grunwell J.R., Stephenson S.T., Tirouvanziam R., Brown L.A.S., Brown M.R., Fitzpatrick A.M. Children with neutrophil-predominant severe asthma have proinflammatory neutrophils with enhanced survival and impaired clearance. J Allergy Clin Immunol Pract. 2019;7:516–525.e6. doi: 10.1016/j.jaip.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor S.L., Leong L.E.X., Choo J.M., Wesselingh S., Yang I.A., Upham J.W., Reynolds P.N., Hodge S., James A.L., Jenkins C., Peters M.J., Baraket M., Marks G.B., Gibson P.G., Simpson J.L., Rogers G.B. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J. Allergy Clin. Immunol. 2018;141:94–103.e15. doi: 10.1016/j.jaci.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 122.Zhu J., Message S.D., Qiu Y., Mallia P., Kebadze T., Contoli M., Ward C.K., Barnathan E.S., Mascelli M.A., Kon O.M., Papi A., Stanciu L.A., Jeffery P.K., Johnston S.L. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;145:1219–1229. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guiddir T., Saint-Pierre P., Purenne-Denis E., Lambert N., Laoudi Y., Couderc R., Gouvis-Echraghi R., Amat F., Just J. Neutrophilic steroid-refractory recurrent wheeze and eosinophilic steroid-refractory asthma in children. J Allergy Clin Immunol Pract. 2017;5:1351–1361.e2. doi: 10.1016/j.jaip.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 124.Nakagome K., Nagata M. Involvement and possible role of eosinophils in asthma exacerbation. Front. Immunol. 2018;9:2220. doi: 10.3389/fimmu.2018.02220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hatchwell L., Collison A., Girkin J., Parsons K., Li J., Zhang J., Phipps S., Knight D., Bartlett N.W., Johnston S.L., Foster P.S., Wark P.A.B., Mattes J. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax. 2015;70:854–861. doi: 10.1136/thoraxjnl-2014-205465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fleming L., Wilson N., Regamey N., Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2012;67:193–198. doi: 10.1136/thx.2010.156836. [DOI] [PubMed] [Google Scholar]
- 127.Teague W.G. Blood eosinophilia may not adequately estimate lung fluid eosinophilia in childhood asthma. J. Allergy Clin. Immunol. Pract. 2019;7:2497–2498. doi: 10.1016/j.jaip.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 128.Nagakumar P., Puttur F., Gregory L.G., Denney L., Fleming L., Bush A., Lloyd C.M., Saglani S. Pulmonary type-2 innate lymphoid cells in paediatric severe asthma: phenotype and response to steroids. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.01809-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saglani S., Bush A., Carroll W., Cunningham S., Fleming L., Gaillard E., Gupta A., Murray C., Nagakumar P., Paton J., Roberts G., Seddon P., Sinha I. Biologics for paediatric severe asthma: trick or TREAT? Lancet Respir Med. 2019;7:294–296. doi: 10.1016/S2213-2600(19)30045-1. [DOI] [PubMed] [Google Scholar]
- 130.Just J., Gouvis-Echraghi R., Couderc R., Guillemot-Lambert N., Saint-Pierre P. Novel severe wheezy young children phenotypes: boys atopic multiple-trigger and girls nonatopic uncontrolled wheeze. J. Allergy Clin. Immunol. 2012;130:103–110.e8. doi: 10.1016/j.jaci.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 131.Bacharier L.B., Beigelman A., Calatroni A., Jackson D.J., Gergen P.J., O’Connor G.T., Kattan M., Wood R.A., Sandel M.T., Lynch S.V., Fujimura K.E., Fadrosh D.W., Santee C.A., Boushey H., Visness C.M., Gern J.E., NIAID sponsored Inner-City Asthma Consortium Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am. J. Respir. Crit. Care Med. 2019;199:71–82. doi: 10.1164/rccm.201801-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Subrata L.S., Bizzintino J., Mamessier E., Bosco A., McKenna K.L., Wikström M.E., Goldblatt J., Sly P.D., Hales B.J., Thomas W.R., Laing I.A., LeSouëf P.N., Holt P.G. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J. Immunol. 2009;183:2793–2800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]
- 133.Custovic A., Sonntag H.-J., Buchan I.E., Belgrave D., Simpson A., Prosperi M.C.F. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J. Allergy Clin. Immunol. 2015;136:1645–1652.e8. doi: 10.1016/j.jaci.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 134.Illi S., von Mutius E., Lau S., Niggemann B., Grüber C., Wahn U. Multicentre Allergy Study (MAS) group, Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 135.Gabet S., Just J., Couderc R., Bousquet J., Seta N., Momas I. Early polysensitization is associated with allergic multimorbidity in PARIS birth cohort infants. Pediatr. Allergy Immunol. 2016;27:831–837. doi: 10.1111/pai.12622. [DOI] [PubMed] [Google Scholar]
- 136.Hose A.J., Depner M., Illi S., Lau S., Keil T., Wahn U., Fuchs O., Pfefferle P.I., Schmaußer-Hechfellner E., Genuneit J., Lauener R., Karvonen A.M., Roduit C., Dalphin J.-C., Riedler J., Pekkanen J., von Mutius E., Ege M.J., MAS, PASTURE study groups Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J. Allergy Clin. Immunol. 2017;139:1935–1945.e12. doi: 10.1016/j.jaci.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 137.Holt P.G., Mok D., Panda D., Renn L., Fabozzi G., deKlerk N.H., Kusel M.M.H., Serralha M., Hollams E.M., Holt B.J., Sly P.D., Rabin R.L. Developmental regulation of type 1 and type 3 interferon production and risk for infant infections and asthma development. J. Allergy Clin. Immunol. 2019;143:1176–1182.e5. doi: 10.1016/j.jaci.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 138.Custovic A., Belgrave D., Lin L., Bakhsoliani E., Telcian A.G., Solari R., Murray C.S., Walton R.P., Curtin J., Edwards M.R., Simpson A., Rattray M., Johnston S.L. Cytokine responses to rhinovirus and development of asthma, allergic sensitization, and respiratory infections during childhood. Am. J. Respir. Crit. Care Med. 2018;197:1265–1274. doi: 10.1164/rccm.201708-1762OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Donovan B.M., Bastarache L., Turi K.N., Zutter M.M., Hartert T.V. The current state of omics technologies in the clinical management of asthma and allergic diseases. Ann. Allergy Asthma Immunol. 2019;123:550–557. doi: 10.1016/j.anai.2019.08.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Farzan N., Vijverberg S.J., Kabesch M., Sterk P.J., Maitland-van der Zee A.H. The use of pharmacogenomics, epigenomics, and transcriptomics to improve childhood asthma management: Where do we stand? Pediatr. Pulmonol. 2018;53:836–845. doi: 10.1002/ppul.23976. [DOI] [PubMed] [Google Scholar]
- 141.Hall R., Hall I.P., Sayers I. Genetic risk factors for the development of pulmonary disease identified by genome-wide association. Respirology. 2019;24:204–214. doi: 10.1111/resp.13436. [DOI] [PubMed] [Google Scholar]
- 142.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S., von Mutius E., Farrall M., Lathrop M., Cookson W.O.C.M., GABRIEL Consortium A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Altman M.C., Gill M.A., Whalen E., Babineau D.C., Shao B., Liu A.H., Jepson B., Gruchalla R.S., O’Connor G.T., Pongracic J.A., Kercsmar C.M., Khurana Hershey G.K., Zoratti E.M., Johnson C.C., Teach S.J., Kattan M., Bacharier L.B., Beigelman A., Sigelman S.M., Presnell S., Gern J.E., Gergen P.J., Wheatley L.M., Togias A., Busse W.W., Jackson D.J. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat. Immunol. 2019;20:637–651. doi: 10.1038/s41590-019-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bosco A., Ehteshami S., Panyala S., Martinez F.D. Interferon regulatory factor 7 is a major hub connecting interferon-mediated responses in virus-induced asthma exacerbations in vivo. J. Allergy Clin. Immunol. 2012;129:88–94. doi: 10.1016/j.jaci.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aoki T., Matsumoto Y., Hirata K., Ochiai K., Okada M., Ichikawa K., Shibasaki M., Arinami T., Sumazaki R., Noguchi E. Expression profiling of genes related to asthma exacerbations. Clin. Exp. Allergy. 2009;39:213–221. doi: 10.1111/j.1365-2222.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 146.Persson H., Kwon A.T., Ramilowski J.A., Silberberg G., Söderhäll C., Orsmark-Pietras C., Nordlund B., Konradsen J.R., de Hoon M.J.L., Melén E., Hayashizaki Y., Hedlin G., Kere J., Daub C.O. Transcriptome analysis of controlled and therapy-resistant childhood asthma reveals distinct gene expression profiles. J. Allergy Clin. Immunol. 2015;136:638–648. doi: 10.1016/j.jaci.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 147.Fitzpatrick A.M., Park Y., Brown L.A.S., Jones D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J. Allergy Clin. Immunol. 2014;133 doi: 10.1016/j.jaci.2013.10.012. 258-261.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chiu C.-Y., Cheng M.-L., Chiang M.-H., Kuo Y.-L., Tsai M.-H., Chiu C.-C., Lin G. Gut microbial-derived butyrate is inversely associated with IgE responses to allergens in childhood asthma. Pediatr. Allergy Immunol. 2019;30:689–697. doi: 10.1111/pai.13096. [DOI] [PubMed] [Google Scholar]
- 149.Duffy K.E., Lamb R.J., San Mateo L.R., Jordan J.L., Canziani G., Brigham-Burke M., Korteweg J., Cunningham M., Beck H.S., Carton J., Giles-Komar J., Duchala C., Sarisky R.T., Mbow M.L. Down modulation of human TLR3 function by a monoclonal antibody. Cell. Immunol. 2007;248:103–114. doi: 10.1016/j.cellimm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 150.Silkoff P.E., Flavin S., Gordon R., Loza M.J., Sterk P.J., Lutter R., Diamant Z., Turner R.B., Lipworth B.J., Proud D., Singh D., Eich A., Backer V., Gern J.E., Herzmann C., Halperin S.A., Mensinga T.T., Del Vecchio A.M., Branigan P., San Mateo L., Baribaud F., Barnathan E.S., Johnston S.L. Toll-like receptor 3 blockade in rhinovirus-induced experimental asthma exacerbations: a randomized controlled study. J. Allergy Clin. Immunol. 2018;141:1220–1230. doi: 10.1016/j.jaci.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 151.Wong J.P., Christopher M.E., Viswanathan S., Karpoff N., Dai X., Das D., Sun L.Q., Wang M., Salazar A.M. Activation of toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine. 2009;27:3481–3483. doi: 10.1016/j.vaccine.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tian X., Xu F., Lung W.Y., Meyerson C., Ghaffari A.A., Cheng G., Deng J.C. Poly I: C enhances susceptibility to secondary pulmonary infections by gram-positive bacteria. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0041879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Torres D., Dieudonné A., Ryffel B., Vilain E., Si-Tahar M., Pichavant M., Lassalle P., Trottein F., Gosset P. Double-stranded RNA exacerbates pulmonary allergic reaction through TLR3: implication of airway epithelium and dendritic cells. J. Immunol. 2010;185:451–459. doi: 10.4049/jimmunol.0902833. [DOI] [PubMed] [Google Scholar]
- 154.Abrams E.M., Raissy H.H. Emerging therapies in the treatment of early childhood wheeze. Pediatr Allergy Immunol Pulmonol. 2019;32:78–80. doi: 10.1089/ped.2019.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cazzola M., Anapurapu S., Page C.P. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: a meta-analysis. Pulm. Pharmacol. Ther. 2012;25:62–68. doi: 10.1016/j.pupt.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 156.Gutiérrez-Tarango M.D., Berber A. Safety and efficacy of two courses of OM-85 BV in the prevention of respiratory tract infections in children during 12 months. Chest. 2001;119:1742–1748. doi: 10.1378/chest.119.6.1742. [DOI] [PubMed] [Google Scholar]
- 157.Schaad U.B. OM-85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J. Pediatr. 2010;6:5–12. doi: 10.1007/s12519-010-0001-x. [DOI] [PubMed] [Google Scholar]
- 158.Collet J.P., Ducruet T., Kramer M.S., Haggerty J., Floret D., Chomel J.J., Durr F. Stimulation of nonspecific immunity to reduce the risk of recurrent infections in children attending day-care centers. The Epicrèche Research Group. Pediatr. Infect. Dis. J. 1993;12:648–652. doi: 10.1097/00006454-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 159.Yin J., Xu B., Zeng X., Shen K. Broncho-Vaxom in pediatric recurrent respiratory tract infections: A systematic review and meta-analysis. Int. Immunopharmacol. 2018;54:198–209. doi: 10.1016/j.intimp.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 160.Ricci R., Palmero C., Bazurro G., Riccio A.M., Garelli V., Di Marco E., Cirillo C., Braido F., Canonica G.W., Melioli G. The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulm. Pharmacol. Ther. 2014;27:109–113. doi: 10.1016/j.pupt.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 161.Emeryk A., Bartkowiak-Emeryk M., Raus Z., Braido F., Ferlazzo G., Melioli G. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children-The EOLIA study. Pediatr. Allergy Immunol. 2018;29:394–401. doi: 10.1111/pai.12894. [DOI] [PubMed] [Google Scholar]
- 162.Razi C.H., Harmancı K., Abacı A., Özdemir O., Hızlı S., Renda R., Keskin F. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J. Allergy Clin. Immunol. 2010;126:763–769. doi: 10.1016/j.jaci.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 163.Oral Bacterial Extract for the Prevention of Wheezing Lower Respiratory Tract Illness - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT02148796 (accessed February 9, 2020).
- 164.Huber M., Mossmann H., Bessler W.G. Th1-orientated immunological properties of the bacterial extract OM-85-BV. Eur. J. Med. Res. 2005;10:209–217. [PubMed] [Google Scholar]
- 165.Zelle-Rieser C., Ramoner R., Bartsch G., Thurnher M. A clinically approved oral vaccine against pneumotropic bacteria induces the terminal maturation of CD83+ immunostimulatory dendritic cells. Immunol. Lett. 2001;76:63–67. doi: 10.1016/s0165-2478(00)00326-6. [DOI] [PubMed] [Google Scholar]
- 166.Mauël J. Stimulation of immunoprotective mechanisms by OM-85 BV. Res. 1994;61:8–15. doi: 10.1159/000196372. [DOI] [PubMed] [Google Scholar]
- 167.Sousa F.H., Casanova V., Findlay F., Stevens C., Svoboda P., Pohl J., Proudfoot L., Barlow P.G. Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides. 2017;95:76–83. doi: 10.1016/j.peptides.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y., Chu X., Liu C., Huang W., Yao Y., Xia Y., Sun P., Long Q., Feng X., Li K., Yang X., Bai H., Sun W., Ma Y. Exogenous murine antimicrobial peptide CRAMP significantly exacerbates Ovalbumin-induced airway inflammation but ameliorates oxazolone-induced intestinal colitis in BALB/c mice. Hum. Vaccin. Immunother. 2018;14:146–158. doi: 10.1080/21645515.2017.1386823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Telcian A.G., Zdrenghea M.T., Edwards M.R., Laza-Stanca V., Mallia P., Johnston S.L., Stanciu L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93–101. doi: 10.1016/j.antiviral.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 170.Greiller C.L., Suri R., Jolliffe D.A., Kebadze T., Hirsman A.G., Griffiths C.J., Johnston S.L., Martineau A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019;187:152–159. doi: 10.1016/j.jsbmb.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 171.Schögler A., Muster R.J., Kieninger E., Casaulta C., Tapparel C., Jung A., Moeller A., Geiser T., Regamey N., Alves M.P. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 2016;47:520–530. doi: 10.1183/13993003.00665-2015. [DOI] [PubMed] [Google Scholar]
- 172.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Stelmach I., Kumar G.T., Urashima M., Camargo C.A. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sandhu M.S., Casale T.B. The role of vitamin D in asthma. Ann. Allergy Asthma Immunol. 2010;105(3):191–199. doi: 10.1016/j.anai.2010.01.013. quiz 200–202. [DOI] [PubMed] [Google Scholar]
- 174.Wang T.-T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S., White J.H., Hanrahan J.H. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 175.Kim V., Abreu R.M., Nakagawa D.M., Baldassare R.M., Carrilho F.J., Ono S.K. Pegylated interferon alfa for chronic hepatitis B: systematic review and meta-analysis. J. Viral Hepat. 2016;23:154–169. doi: 10.1111/jvh.12418. [DOI] [PubMed] [Google Scholar]
- 176.Lavender K.J., Gibbert K., Peterson K.E., Van Dis E., Francois S., Woods T., Messer R.J., Gawanbacht A., Müller J.A., Münch J., Phillips K., Race B., Harper M.S., Guo K., Lee E.J., Trilling M., Hengel H., Piehler J., Verheyen J., Wilson C.C., Santiago M.L., Hasenkrug K.J., Dittmer U. Interferon alpha subtype-specific suppression of HIV-1 infection in vivo. J. Virol. 2016;90:6001–6013. doi: 10.1128/JVI.00451-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Trang T.P., Whalen M., Hilts-Horeczko A., Doernberg S.B., Liu C. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: a single-center retrospective cohort study and review of the literature. Transpl. Infect. Dis. 2018;20 doi: 10.1111/tid.12844. [DOI] [PubMed] [Google Scholar]
- 178.Grim S.A., Reid G.E., Clark N.M. Update in the treatment of non-influenza respiratory virus infection in solid organ transplant recipients. Expert Opin. Pharmacother. 2017;18:767–779. doi: 10.1080/14656566.2017.1322063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ruuskanen O., Waris M., Kainulainen L. Treatment of persistent rhinovirus infection with pegylated interferon α2a and ribavirin in patients with hypogammaglobulinemia. Clin. Infect. Dis. 2014;58:1784–1786. doi: 10.1093/cid/ciu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wong S.S.Y., Yuen K.-Y. The management of coronavirus infections with particular reference to SARS. J. Antimicrob. Chemother. 2008;62:437–441. doi: 10.1093/jac/dkn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Becker T.M., Durrani S.R., Bochkov Y.A., Devries M.K., Rajamanickam V., Jackson D.J. Effect of exogenous interferons on rhinovirus replication and airway inflammatory responses. Ann. Allergy Asthma Immunol. 2013;111:397–401. doi: 10.1016/j.anai.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Djukanović R., Harrison T., Johnston S.L., Gabbay F., Wark P., Thomson N.C., Niven R., Singh D., Reddel H.K., Davies D.E., Marsden R., Boxall C., Dudley S., Plagnol V., Holgate S.T., Monk P., INTERCIA Study Group The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am. J. Respir. Crit. Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.C. McCrae, M. Olsson, M. Aurell, C. Lundin, J. Paraskos, A. Cavallin, M. Kjerrulf, K. Karlsson, R. Marsden, A. Malmgren, P. Gustafson, T. Harrison, On-demand inhaled interferon-beta 1a for the prevention of severa asthma exacerbations: results of the INEXAS phase 2a study, in: D12. Immunotherapy in Lung Disease, American Thoracic Society, 2018: pp. A6165–A6165. 10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A6165. [DOI]
- 184.A Study in Asthma Patients to Evaluate Efficacy, Safety and Tolerability of 14 Days Once Daily Inhaled Interferon Beta-1a After the Onset of Symptoms of an Upper Respiratory Tract Infection - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT02491684 (accessed February 4, 2020).
- 185.Thibaut H.J., De Palma A.M., Neyts J. Combating enterovirus replication: state-of-the-art on antiviral research. Biochem. Pharmacol. 2012;83:185–192. doi: 10.1016/j.bcp.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 186.Effects of Pleconaril Nasal Spray on Common Cold Symptoms and Asthma Exacerbations Following Rhinovirus Exposure (Study P04295) - Study Results - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/results/NCT00394914 (accessed May 3, 2020).
- 187.Potaczek D.P., Unger S.D., Zhang N., Taka S., Michel S., Akdağ N., Lan F., Helfer M., Hudemann C., Eickmann M., Skevaki C., Megremis S., Sadewasser A., Alashkar Alhamwe B., Alhamdan F., Akdis M., Edwards M.R., Johnston S.L., Akdis C.A., Becker S., Bachert C., Papadopoulos N.G., Garn H., Renz H. Development and characterization of DNAzyme candidates demonstrating significant efficiency against human rhinoviruses. J. Allergy Clin. Immunol. 2019;143:1403–1415. doi: 10.1016/j.jaci.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 188.Bush A. Azithromycin is the answer in paediatric respiratory medicine, but what was the question? Paediatr. Respir. Rev. 2019 doi: 10.1016/j.prrv.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 189.de Benedictis F.M., Carloni I., Guidi R. Question 4: Is there a role for antibiotics in infantile wheeze? Paediatr. Respir. Rev. 2019 doi: 10.1016/j.prrv.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 190.Li P., Zhang T., Xiao Y., Tian L., Cui B., Ji G., Liu Y.-Y., Zhang F. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl. Microbiol. Biotechnol. 2019;103:349–360. doi: 10.1007/s00253-018-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang H.T., Anvari S., Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel) 2019;6 doi: 10.3390/children6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cuello-Garcia C., Fiocchi A., Pawankar R., Yepes-Nuñez J.J., Morgano G.P., Zhang Y., Agarwal A., Gandhi S., Terracciano L., Schünemann H.J., Brozek J.L. Prebiotics for the prevention of allergies: a systematic review and meta-analysis of randomized controlled trials. Clin. Exp. Allergy. 2017;47:1468–1477. doi: 10.1111/cea.13042. [DOI] [PubMed] [Google Scholar]
- 193.Thorburn A.N., McKenzie C.I., Shen S., Stanley D., Macia L., Mason L.J., Roberts L.K., Wong C.H.Y., Shim R., Robert R., Chevalier N., Tan J.K., Mariño E., Moore R.J., Wong L., McConville M.J., Tull D.L., Wood L.G., Murphy V.E., Mattes J., Gibson P.G., Mackay C.R. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 194.Schuijt T.J., Lankelma J.M., Scicluna B.P., de Sousa e Melo F., Roelofs J.J.T.H., de Boer J.D., Hoogendijk A.J., de Beer R., de Vos A., Belzer C., de Vos W.M., van der Poll T., Wiersinga W.J. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sencio V., Barthelemy A., Tavares L.P., Machado M.G., Soulard D., Cuinat C., Queiroz-Junior C.M., Noordine M.-L., Salomé-Desnoulez S., Deryuter L., Foligné B., Wahl C., Frisch B., Vieira A.T., Paget C., Milligan G., Ulven T., Wolowczuk I., Faveeuw C., Le Goffic R., Thomas M., Ferreira S., Teixeira M.M., Trottein F. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30 doi: 10.1016/j.celrep.2020.02.013. 2934-2947.e6. [DOI] [PubMed] [Google Scholar]
- 196.Brown R.L., Sequeira R.P., Clarke T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017;8:1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schwerk N., Brinkmann F., Soudah B., Kabesch M., Hansen G. Wheeze in preschool age is associated with pulmonary bacterial infection and resolves after antibiotic therapy. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0027913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front. Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bacharier L.B., Guilbert T.W., Mauger D.T., Boehmer S., Beigelman A., Fitzpatrick A.M., Jackson D.J., Baxi S.N., Benson M., Burnham C.-A.D., Cabana M., Castro M., Chmiel J.F., Covar R., Daines M., Gaffin J.M., Gentile D.A., Holguin F., Israel E., Kelly H.W., Lazarus S.C., Lemanske R.F., Ly N., Meade K., Morgan W., Moy J., Olin T., Peters S.P., Phipatanakul W., Pongracic J.A., Raissy H.H., Ross K., Sheehan W.J., Sorkness C., Szefler S.J., Teague W.G., Thyne S., Martinez F.D. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stokholm J., Chawes B.L., Vissing N.H., Bjarnadóttir E., Pedersen T.M., Vinding R.K., Schoos A.-M.M., Wolsk H.M., Thorsteinsdóttir S., Hallas H.W., Arianto L., Schjørring S., Krogfelt K.A., Fischer T.K., Pipper C.B., Bønnelykke K., Bisgaard H. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.P.J. Mandhane, P. Paredes Zambrano de Silbernagel, Y.N. Aung, J. Williamson, B.E. Lee, S. Spier, M. Noseworthy, W.R. Craig, D.W. Johnson, Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: a placebo-controlled randomized trial, PLoS ONE. 12 (2017) e0182411. 10.1371/journal.pone.0182411. [DOI] [PMC free article] [PubMed]
- 202.Pincheira M.A., Bacharier L.B., Castro-Rodriguez J.A. Efficacy of macrolides on acute asthma or wheezing exacerbations in children with recurrent wheezing: a systematic review and meta-analysis. Paediatr. Drugs. 2020 doi: 10.1007/s40272-019-00371-5. [DOI] [PubMed] [Google Scholar]
- 203.Brusselle G.G., Vanderstichele C., Jordens P., Deman R., Slabbynck H., Ringoet V., Verleden G., Demedts I.K., Verhamme K., Delporte A., Demeyere B., Claeys G., Boelens J., Padalko E., Verschakelen J., Van Maele G., Deschepper E., Joos G.F.P. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 204.Gibson P.G., Yang I.A., Upham J.W., Reynolds P.N., Hodge S., James A.L., Jenkins C., Peters M.J., Marks G.B., Baraket M., Powell H., Taylor S.L., Leong L.E.X., Rogers G.B., Simpson J.L. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 205.Hahn D.L., Grasmick M., Hetzel S., Yale S., AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group Azithromycin for bronchial asthma in adults: an effectiveness trial. J. Am. Board Fam. Med. 2012;25:442–459. doi: 10.3122/jabfm.2012.04.110309. [DOI] [PubMed] [Google Scholar]
- 206.Hiles S.A., McDonald V.M., Guilhermino M., Brusselle G.G., Gibson P.G. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.01381-2019. [DOI] [PubMed] [Google Scholar]
- 207.Simpson J.L., Powell H., Boyle M.J., Scott R.J., Gibson P.G. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am. J. Respir. Crit. Care Med. 2008;177:148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 208.Yamaya M., Nomura K., Arakawa K., Sugawara M., Deng X., Lusamba Kalonji N., Nishimura H., Yamada M., Nagatomi R., Kawase T. Clarithromycin decreases rhinovirus replication and cytokine production in nasal epithelial cells from subjects with bronchial asthma: effects on IL-6, IL-8 and IL-33. Arch. Pharm. Res. 2017 doi: 10.1007/s12272-017-0950-x. [DOI] [PubMed] [Google Scholar]
- 209.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 210.Busse W.W., Morgan W.J., Gergen P.J., Mitchell H.E., Gern J.E., Liu A.H., Gruchalla R.S., Kattan M., Teach S.J., Pongracic J.A., Chmiel J.F., Steinbach S.F., Calatroni A., Togias A., Thompson K.M., Szefler S.J., Sorkness C.A. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Deschildre A., Marguet C., Salleron J., Pin I., Rittié J.-L., Derelle J., Taam R.A., Fayon M., Brouard J., Dubus J.C., Siret D., Weiss L., Pouessel G., Beghin L., Just J. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur. Respir. J. 2013;42:1224–1233. doi: 10.1183/09031936.00149812. [DOI] [PubMed] [Google Scholar]
- 212.Esquivel A., Busse W.W., Calatroni A., Togias A.G., Grindle K.G., Bochkov Y.A., Gruchalla R.S., Kattan M., Kercsmar C.M., Khurana Hershey G., Kim H., Lebeau P., Liu A.H., Szefler S.J., Teach S.J., West J.B., Wildfire J., Pongracic J.A., Gern J.E. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am. J. Respir. Crit. Care Med. 2017;196:985–992. doi: 10.1164/rccm.201701-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kantor D.B., McDonald M.C., Stenquist N., Schultz B.J., Smallwood C.D., Nelson K.A., Phipatanakul W., Hirschhorn J.N. Omalizumab is associated with reduced acute severity of rhinovirus-triggered asthma exacerbation. Am. J. Respir. Crit. Care Med. 2016;194:1552–1555. doi: 10.1164/rccm.201606-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Heymann P.W., Platts-Mills T.A., Woodfolk J.A., Borish L., Murphy D.D., Carper H.T., Conaway M.R., Steinke J.W., Muehling L., Gerald T.W., Kennedy J.L., Irani A.-M., McGraw M.D., Early S.V., Wheatley L.M., Adams A.P., Turner R.B. Understanding the asthmatic response to an experimental rhinovirus infection: exploring the effects of blocking IgE. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Teach S.J., Gill M.A., Togias A., Sorkness C.A., Arbes S.J., Calatroni A., Wildfire J.J., Gergen P.J., Cohen R.T., Pongracic J.A., Kercsmar C.M., Khurana Hershey G.K., Gruchalla R.S., Liu A.H., Zoratti E.M., Kattan M., Grindle K.A., Gern J.E., Busse W.W., Szefler S.J. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015;136:1476–1485. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gill M.A., Liu A.H., Calatroni A., Krouse R.Z., Shao B., Schiltz A., Gern J.E., Togias A., Busse W.W. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J. Allergy Clin. Immunol. 2018;141:1735–1743.e9. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Just J., Deschildre A., Lejeune S., Amat F. New perspectives of childhood asthma treatment with biologics. Pediatr. Allergy Immunol. 2019;30:159–171. doi: 10.1111/pai.13007. [DOI] [PubMed] [Google Scholar]
- 218.Gupta A., Ikeda M., Geng B., Azmi J., Price R.G., Bradford E.S., Yancey S.W., Steinfeld J. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J. Allergy Clin. Immunol. 2019;144:1336–1342.e7. doi: 10.1016/j.jaci.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 219.Sabogal Piñeros Y.S., Bal S.M., van de Pol M.A., Dierdorp B.S., Dekker T., Dijkhuis A., Brinkman P., van der Sluijs K.F., Zwinderman A.H., Majoor C.J., Bonta P.I., Ravanetti L., Sterk P.J., Lutter R. Anti-IL-5 in mild asthma alters rhinovirus-induced macrophage, B-cell, and neutrophil responses (MATERIAL). A placebo-controlled, double-blind study. Am. J. Respir. Crit. Care Med. 2019;199:508–517. doi: 10.1164/rccm.201803-0461OC. [DOI] [PubMed] [Google Scholar]