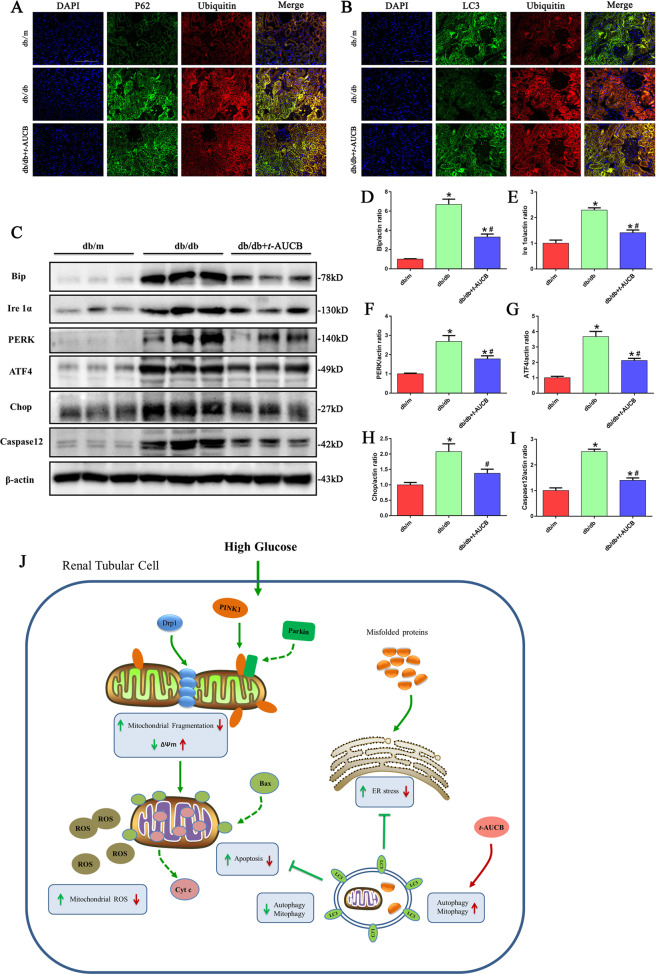
Fig. 8. t-AUCB administration attenuated ER stress in db/db mice.
a, b Representative images of immunofluorescence double labeling of p62 and Ubiquitin as well as LC3 and Ubiquitin in different kidney tissues. c Western blot analysis of Bip, Ire1α, PERK, ATF4, Chop and Caspase12 expression in kidney tissues. d–i Densitometric analysis of Bip, Ire1α, PERK, ATF4, Chop and Caspase12 expression in figure c. j Schematic diagram depicting inhibition of soluble epoxide hydrolase with t-AUCB attenuates renal tubular mitochondrial dysfunction and ER stress by restoring autophagic flux in diabetic nephropathy. HG exposure induced mitochondrial dysfunction, and ER stress could then lead to cell apoptosis and injury. Under normal conditions, autophagy serves as an adaptive mechanism to eliminate damaged mitochondria (mitophagy) and misfolded proteins to maintain mitochondrial quality and alleviate ER stress. However, under HG conditions, both autophagy and mitophagy are suppressed, and a compromise in the process of PINK1/Parkin -mediated mitophagy results in insufficient autophagic removal of mitochondria, which eventually leads to the accumulation of fragmented and damaged mitochondria, and subsequently triggers ROS overproduction and activation of the mitochondrial apoptotic pathway. Meanwhile, impaired autophagy hinders autophagic function as a compensatory mechanism to remove misfolded protein aggregates, which enhances the accumulation of ubiquitinated protein and aggravates ER stress. Interestingly, pharmacologic inhibition of sEH by t-AUCB restores impaired autophagy flux, which subsequently attenuates renal tubular mitochondrial dysfunction and ER stress, thereby alleviating hyperglycemia-induced proximal renal tubular injury.