Abstract
Facilitation studies typically compare plants under differential stress levels with and without neighbors, while the density of neighbors has rarely been addressed. However, recent empirical studies indicate that facilitation may be density-dependent too and peak at intermediate neighbor densities. Here, we propose a conceptual model to incorporate density-dependence into theory about changes of plant–plant interactions under stress. To test our predictions, we combine an individual-based model incorporating both facilitative response and effect, with an experiment using salt stress and Arabidopsis thaliana. Theoretical and experimental results are strikingly consistent: (1) the intensity of facilitation peaks at intermediate density, and this peak shifts to higher densities with increasing stress; (2) this shift further modifies the balance between facilitation and competition such that the stress-gradient hypothesis applies only at high densities. Our model suggests that density-dependence must be considered for predicting plant–plant interactions under environmental change.
Subject terms: Ecology, Community ecology, Ecological modelling, Population dynamics
Density-dependence is rarely accounted for in plant-plant facilitation studies. Here the authors develop a framework that incorporates density-dependence in the stress-gradient hypothesis, and test it first through modeling and then empirically on Arabidopsis thaliana along salt stress gradients.
Introduction
Competitive interactions and environmental stress are crucial factors for structuring plant communities and shaping species distributions1,2. Though competition dominates the literature, positive plant–plant interactions (facilitation) have been widely explored in the past two decades. For example, facilitation may affect species distribution and range shifts with climate change3,4 and/or plant diversity from local to large scales5. However, most facilitation research is about specific empirical case studies, while theoretical progress helping to generalize the findings has far lagged behind. The most influential model of facilitation, stress-gradient hypothesis (SGH), which predicts dominance of facilitation over competition under intense stress6, is conceptual instead of quantitative and there is still much debate about its generality7–15. Apparently, this hinders our ability to connect plant interactions with environmental change, thus hampering efforts to predict the response of populations and communities to pressures and disturbance under ongoing global change16–18.
One reason why the theoretical progress has lagged behind could be that most facilitation studies are empirical and only look at target plants under two conditions—with and without neighbors, while the quantity of neighbors (i.e., their density), has been largely ignored. Density dependence has been paramount for theoretical studies of competition and associated fundamental rules such as species coexistence19–21, the law of constant final yield22, or self-thinning23. It is therefore surprising that facilitation studies rarely address a core aspect of plant interactions i.e., density-dependence11,24–26.
The density of neighbor plants should have major effects on facilitation27. For example, the magnitude of facilitation could increase with benefactor densities, if the net effect of one benefactor is positive. In other words, low densities may be insufficient to alleviate stress in the neighborhood effectively28,29. However, stress amelioration is not unlimited (e.g., when neighborhood has been fully shaded by neighbors under hot and dry conditions). Also considering that facilitative and competitive interactions usually co-occur27,30, then facilitation should be superseded by competition at very high densities. Consequently, we may expect a non-linear relationship between density and net facilitative effects.
Recent empirical studies support this idea and show that benefactors exert the strongest facilitative effect at intermediate densities28,31–33. However, few existing studies have attempted to explicitly link density-dependence with changes of plant interactions along stress gradients. Facilitation involves benefactors and beneficiaries, i.e., stress amelioration by benefactors (facilitative effect) and sensitivity of beneficiaries to this amelioration (facilitative response) are equally important34. Nevertheless, most studies focus either on beneficiaries or on benefactors alone35,36 and so are the few studies investigating density-dependence24,27.
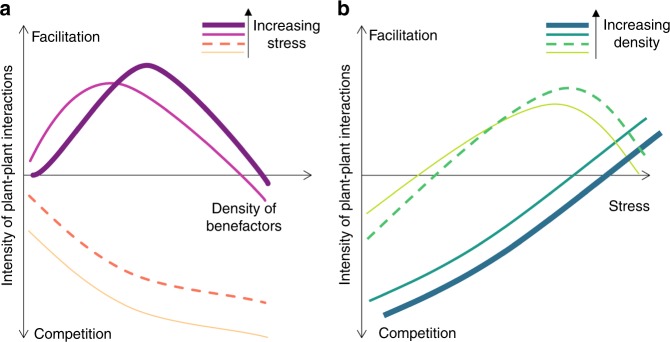
The facilitative response of a beneficiary is determined by the distance to its ecological optimum17,36,37, i.e., plants are more sensitive to facilitation under intensive stress. Thus, the facilitation–density curve should move upward with increasing stress (Fig. 1a), unless stress exceeds physiological tolerance of beneficiaries. On the other hand, benefactors also experience stress, which may strongly weaken their own performance (e.g., size) and beneficial effects11,26,38. We may assume that higher densities compensate the reduced positive effects of smaller (or fewer) beneficiaries, i.e., more neighbors are needed at higher stress levels for maintaining a given level of habitat amelioration. Accordingly, the peak of facilitation will occur at higher densities, i.e., with intensifying stress, the unimodal curve moves to the right along a density gradient (Fig. 1a). This further suggests that at low densities, the net outcome will be less positive and the SGH may not apply (Fig. 1b).
Fig. 1. The change of plant–plant interactions under stress.
a How interaction intensity–density relationships should shift along a stress gradient and how the shift may affect the balance between facilitation and competition. Under low density (left of the intersection between the two highest stress levels), interactions become less negative with increasing stress but are not most positive for the highest stress level, whereas under high density, the shift in interaction intensity is continuous and follows the predictions of the stress-gradient hypothesis. b Same model as panel a but displaying how plant–plant interactions at differential densities change along a stress gradient. This shows that the SGH applies only to relatively high densities.
Here, we combine a mathematical model with highly controlled experiments to test these predictions in an intraspecific setting. Classical theoretical studies of negative density-dependence, such as self-thinning22,23, have been based on a population level. Therefore, any attempt to integrate classical competition theory with facilitation should not avoid the intraspecific setting. Indeed, facilitation may be observed less easily among conspecifics due to larger niche overlap19,20 and stronger intraspecific competition. However, the advantage is that it could provide a general framework, which is unconfounded by species-specifity of facilitative effect and response34,39. Namely, (conspecific) benefactors and beneficiaries do not differ in their niches and are affected similarly by any given stress factor and vice-versa, share the very same resources.
Our individual-based model is designed to provide generic insights into the link between plant interactions and environmental change. The few existing models31,40 focus narrowly on facilitative effect, while differential response has rarely been considered simultaneously. However, as a pairwise interaction, facilitation should be determined by both of them. Recent empirical studies have also showed that even conspecifics could differ greatly in their response, with smaller ones being more sensitive than larger individuals41,42. Moreover, neglecting facilitative response has led to a mathematical problem, where the units on the two sides of their equations are not identical (see “Methods”). By integrating both elements, our model is able to predict the change of plant interactions under stress more explicitly. Though our model is designed to be very general, we also test whether this pattern can be reproduced with real plants. To that end, we conduct a parallel greenhouse experiment with the model plant Arabidopsis thaliana which are grown in a full-factorial setting along both a density and a salinity gradient. Salinity is selected as the stress factor, because it is easy to manipulate quantitatively34 and has been well studied29,34,43.
We first examine whether facilitation follows the hypothesized hump-shaped relationship. Then we test the following predictions: (1) With increasing stress, facilitation peaks at higher densities. (2) This rightward shift along the density axis changes the balance between facilitation and competition i.e., the SGH holds at high but not at low densities. Results of model simulations and the experiment are strikingly similar and they strongly corroborate these predictions, suggesting the change of plant–plant interactions along stress gradients can be predictable, but only when density is considered explicitly. These findings also indicate the importance of including density-dependence in models for understanding the response of plant populations and communities to environmental change.
Results
Density-dependence of plant–plant interactions
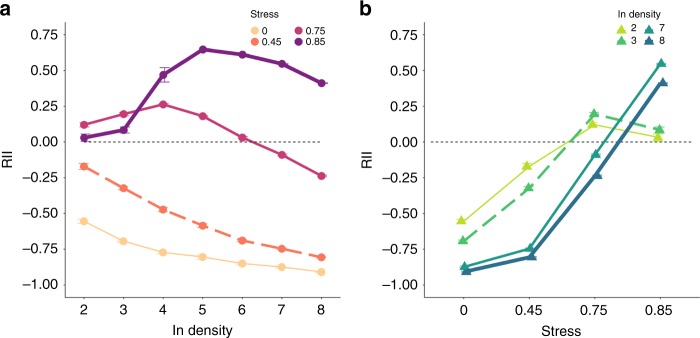
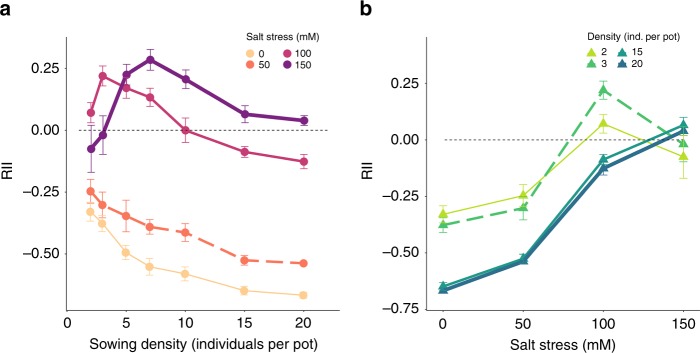
Changes of relative interaction indexes (RIIs) indicated that the net outcome of plant–plant interactions was strongly affected both by stress and density. Note that RII is used to quantify the strength of net plant interactions, which ranges from −1 to 1 with negative values indicating competition and positive values net facilitative interactions (see “Methods“). In model simulations, the relationship between RIIs and density changed from monotonically decreasing to hump-shaped with increasing stress (Fig. 2; see Supplementary Fig. 1 for more stress levels). The experimental results, i.e., from a linear to a hump-shaped relationship, were strikingly similar to those of the model (Fig. 3). Similar patterns were also found for survival and fecundity (Supplementary Figs. 2, 3).
Fig. 2. The change of density-dependent interactions under stress in model simulations.
a Relationship between initial density and relative interaction index (RII; data are presented as mean values ± SEM) in simulated populations growing along a stress gradient. b Plant–plant interactions change along the stress gradient, showing that the SGH applies at high densities but not at low densities. Circles represent different stress levels while triangles represent different density levels. N = 5 independent samples in model simulations for the density gradient from two to eight at each stress level. Note that for facilitating visual interpretation, we only show the two lowest and two highest densities (changes in the RII for intermediate densities were similar and ranged in between the extreme scenarios shown here). Source data are provided as a Source Data file.
Fig. 3. The change of density-dependent interactions under salt stress in the experiment.
a Relationship between initial density and average relative interaction index (RII; data are presented as mean values ± SEM) for biomass of Arabidopsis thaliana grown along a salinity gradient in a greenhouse experiment. b Plant–plant interactions change along the stress gradient, showing that the SGH applies at high densities but not at low densities. Circles represent different stress levels while triangles and lines represent different density levels. For the density gradient from 2 to 20 plants per pot, nsalt_0 = 25, 29, 17, 16, 15, 15, and 16 independent pots, respectively; nsalt_50 = 20, 25, 13, 17, 16, 13, 16; nsalt_100 = 30, 26, 15, 12, 14, 14, 13; nsalt_150 = 29, 30, 15, 16, 17, 12, 11. Note that for facilitating visual interpretation, we only show the two lowest and two highest densities (changes in the RII for intermediate densities were similar and ranged in between the extreme scenarios shown here). Source data are provided as a Source Data file.
Shift of the facilitation–density relationship
With increasing stress, both model and experiment yielded a peak in RII at higher densities. Specifically, the unimodal facilitation–density curve shifted to the high end of the density gradient (Figs. 2a, 3a). Correspondingly, at high densities (e.g., 15 and 20 individuals per pot), net plant–plant interactions shifted from more negative to more positive along the stress gradient (Figs. 2b, 3b). However, at low densities (e.g., two and three individuals per pot), facilitation under high stress was more intense than under extreme stress, i.e., the balance between positive and negative interactions responded in a hump-shape to increasing stress levels (Figs. 2b, 3b).
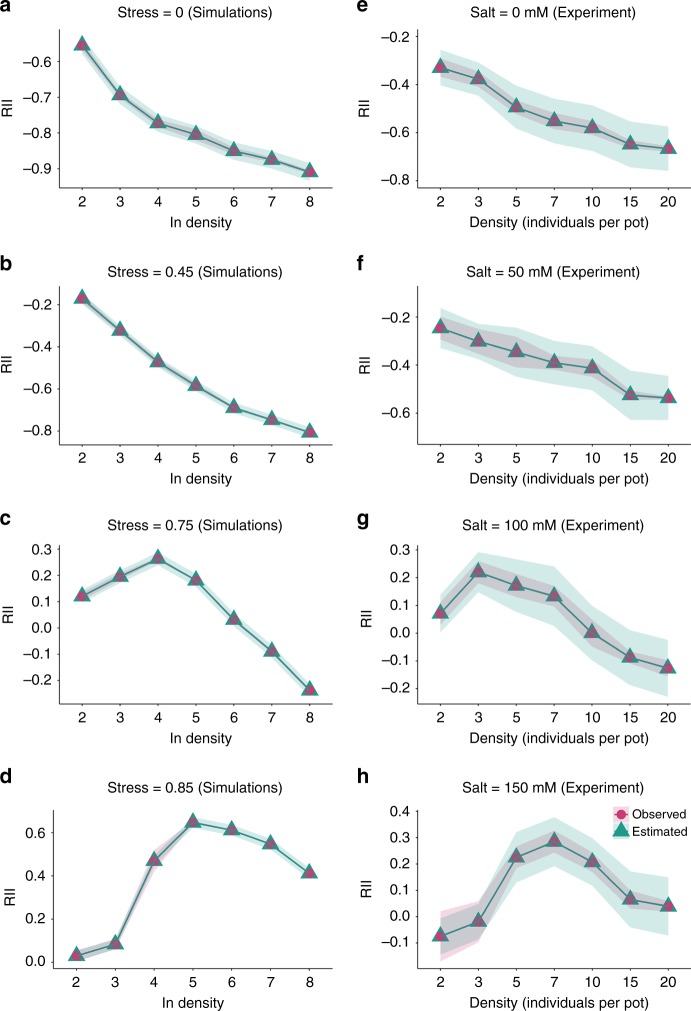
The Bayesian analysis further showed that the full model including density and stress (and their interactions) provided the best-fit to the dataset for both simulations and the experiment (Table 1). There was an approximated probability of 95% for the full model accurately predicting the change of RIIs–density relationship along stress gradients, and this also indicated that the RIIs–density relationship at different stress levels indeed differed greatly (Fig. 4). Furthermore, estimates based on model-averaging were generally similar to the full model. However, its credible sets were larger, reflecting greater uncertainty (Supplementary Fig. 4). The estimated model parameters also fall within the 95% credible sets and they were shown in the Supplementary information (Supplementary Table 1).
Table 1.
Bayesian models relating plant–plant interactions to stress and density.
| Models | WAIC (simulations) | WAIC (experiment) |
|---|---|---|
| RIIs = S | −59.6 | 37.4 |
| RIIs = D | 218.4 | 386.2 |
| RIIs = S + D | −80.5 | −0.1 |
| RIIs = S + D + S × D | −524.4 | −53.8 |
WAIC is used to compare these Bayesian models in model simulations and the experiment, respectively, and the model with smaller WAIC value is considered to be a better model.
RII relative interaction index, S stress in model simulations or salinity level in the experiment, D density, WAIC Watanabe–Akaike information criterion.
Fig. 4. Bayesian estimates and observed plant–plant interactions.
a–d Bayesian estimates (with 95% credible set) and observed average index (RII; with 95% confidence interval) along a density gradient at each stress level in the simulations (n = 5 independent samples in model simulations for the density gradient from two to eight at each stress level). e–h experimental data (for the density gradient from 2 to 20 plants per pot, nsalt_0 = 25, 29, 17, 16, 15, 15 and 16 independent pots, respectively; nsalt_50 = 20, 25, 13, 17, 16, 13, 16; nsalt_100 = 30, 26, 15, 12, 14, 14, 13; nsalt_150 = 29, 30, 15, 16, 17, 12, 11). Circles and represent observed values while triangles and lines represent Bayesian estimates. Source data are provided as a Source Data file.
Effects of spatial patterns, modes, and growth
In additional simulations, the intensity of facilitation at relatively high stress levels was generally stronger with an aggregated spatial pattern than that under regular spacing, but neither spatial pattern nor different modes of competition and facilitation affected the facilitation–density relationship qualitatively (Supplementary Fig. 5). Similarly, the same general pattern was observed when considering the growth of plants, only that the facilitation–density curve shifted leftward along the density gradient over time, i.e., RIIs peaked at lower densities (Supplementary Fig. 6a, b). RIIs generally increased with size (biomass) of plants during growth, except for individuals at relatively high densities. These plants exhibited a unimodal facilitation–size relationship, i.e., facilitation first increased with size while competition dominated as plants grew bigger (Supplementary Fig. 6c, d).
Discussion
Overall findings provide unequivocal support for our initial predictions. There was a predictable, density-dependent relationship between interaction intensity and direction and stress levels, and a predictable shift towards higher densities, which was either consistent or against SGH6. All results were strikingly consistent between the experiment and the model (which incorporated a wide range of scenarios), indicating that they are highly robust.
It is not surprising that monotonous negative density-dependence dominated under relatively benign conditions. This pattern has long been considered universal and is a cornerstone of population biology44. Despite the overwhelming evidence for the ubiquity of positive interactions45, facilitation has been ignored in most models addressing density-dependence. Only few models have considered density-dependence of facilitation31, and despite some limitations (see “Methods”) these corroborate one of our findings, namely that the maximum benefit from neighbors was obtained at intermediate densities. The generally weak positive interactions under low density can be explained by the fact that plants are too remote from each other to affect their neighbors. Though our findings challenge theories based solely on competition22,46, they are consistent with recent empirical studies in stressful habitats. For instance, strong support for a unimodal fecundity–density relationship was found in a Tibetan lotus species32. There was also evidence of density-dependence from interspecific cases, e.g., Dickie et al. found that with increasing density of Quercus ellipsoidalis, the growth of seedlings (Quercus macrocarpa) first increased and then declined33.
Although the hump-shaped response of facilitation to density has been suggested by a few previous studies, confirming this relationship was important because it served as the assumption underlying our main prediction of an upward and rightward shift of the non-linear facilitation–density curve under prevailing stress.
Indeed, RIIs peaked at larger densities with increasing stress (i.e., rightward shift) in both simulations and the experiment. This may be explained by the fact that at higher stress levels, more neighbors are required for ameliorating habitat conditions due to two non-mutually exclusive mechanisms. On the one hand, if a benefactor of a given size alleviates stress to a certain level, then more plants are needed to generate the same conditions in more severe environments. On the other hand, positive effects of benefactors may decline because they themselves are stressed and smaller11,26,38. This could also happen dynamically in time during plant growth. Model simulations showed that facilitation generally increased with plant size during growth, unless there were too many neighbors. Intriguingly, we further found that the facilitation–density curve moved towards the low end of the density gradient over time (i.e., RIIs peaked at lower densities), indicating that fewer plants are needed for ameliorating stressful conditions as plant size and their positive effects increased. Therefore, the dynamics of facilitation also corroborated our hypothesis that the curve should shift rightward when facilitative effects are decreased by stress, abelite indirectly.
If we apply these ideas to our experiments, we must consider that mechanism by which plants facilitate each other under salt stress can be either reduced evaporation via shading of the substrate or salt uptake from the soil29,34. Clearly, this facilitative effect should become stronger with more and/or larger neighbors present, until the vicinity of beneficiaries is fully shaded by canopy or the salt is fully retrieved by neighbors. In our study, performance of all individuals was greatly limited by salinity, and thus more neighbors were needed for alleviating salt stress. Though we tested our model with a single species and stress type (salinity), our simulations provided also the theoretical explanation for studies addressing many other stress factors. It is reasonable to assume that if benefactors ameliorate different stressful conditions, e.g., drought (via shading), disturbance (via substrate stabilization), herbivory (via physical or chemical defense), soil contaminants (via uptake), and many more, then more and/or larger neighbors would yield a larger facilitative effect, too. There are also a few empirical studies exemplifying that our experimental findings are unlikely to be exclusive for salt stress. For example, Goldenheim et al. found for a gradient of evaporative stress that the forb Suaeda linearis switched from negative to positive density-dependence, i.e., in more stressful conditions, plants showed greater biomass and seed production at higher densities due to mitigated desiccation and thermal stress by neighbors47. Similarly, Bos and van Katwijk reported that as hydrodynamic exposure increased, survival of eelgrass Zostera marina was significantly higher in the high-density group because neighbors could reduce drag force when exposed to currents48.
Our second main prediction was that due to the above shift in the unimodal relationship, the balance between competition and facilitation is density-dependent. There was again strikingly consistent support for this hypothesis from the experiment and modeling. Specifically, due to the shift of the facilitation–density curve, the SGH6 was supported for high densities, where interactions changed from predominantly negative to positive with increasing stress. Despite the fact that plant performance was decreased under high densities and intense stress, the number of benefactors was still sufficient for ameliorating the stress, i.e., even the area shaded by each plant was reduced by salt stress in the experiment. However, this pattern was not confirmed under low density and high stress. Under such conditions, not only the facilitative effect of each individual benefactor but also their number was too small. Therefore, initially positive interactions could shift towards neutral or negative.
In fact, many empirical findings have reported the decreased facilitation along stress gradients9–15,26,38. Nevertheless, only case-specific explanations have been proposed and the link to density-dependence has not been made12,14. Indeed, most previous studies merely compared the performance of target plants under two density levels only (with and without neighbors), while models incorporating stress gradients and density-dependence of competition and facilitation are virtually missing. Therefore, our findings may help to provide a quantitative framework for predicting under which conditions the SGH should apply. Such a quantitative framework is urgently needed because there is still no consensus about the generality of the SGH8,25,26,35. This also prevents us from predicting how plant populations and communities may be affected by environmental change17. Here, we demonstrate that by explicitly considering the density of neighbors, it is possible to predict the outcome of plant–plant interactions under different levels of stress.
Our model adopts the idea that facilitation is determined by both facilitative effect and response. However, to our knowledge previous models only considered facilitative effect31,40,49–51. This is regrettable because even for the same species, smaller and/or younger plants respond more positively to stress mitigation than larger ones42. In addition, much accepted theory related to sensitivity to facilitation is also based on species-specific stress tolerance37 which in turn has been related to size in classical plant strategy theory (Grime’s CSR). It is therefore surprising that facilitative response has not been considered in the few existing models. This has also resulted in a mathematical problem, i.e., unbalanced units on the two sides of equations31,40. In fact, these models yield findings which not only contradict our experiments but also cannot be explained. Namely, the facilitation–density relationship moves upward or leftward with increasing stress and one model even indicates strong competition at very low densities under high stress31. This is counterintuitive, and cannot be explained by decreased facilitation intensity with increasing stress at low densities.
It should be noted that while we selected an intraspecific setting for providing a general framework, there could obviously be species-specific differences between benefactors and beneficiaries in interspecific settings, e.g., benefactors and beneficiaries may differ dramatically in stress tolerance, shape and other functional traits17,25. Our model is based on integrating both effect and response because multiple drivers of facilitation could be encapsulated into the two general factors. Therefore, those differences may also determine the shift of facilitation–density relationship, if they could affect the decline of facilitative effects (or response) along stress gradients. For example, benefactor species may be highly tolerant to or even favor, up to a certain point, the very stress factor limiting the beneficiaries39. In this scenario, if net effects of benefactors were not reduced by stress, we would expect that the curve simply moves upward (or perhaps leftward with increased facilitative effects) instead of moving to the right. Furthermore, an interspecific scenario would also differ in the way negative plant–plant interactions play out, i.e., competition should be less intense between than within species19. In this case, the RII–density relationship would again be similar to our intraspecific case, but facilitation may dominate across a larger range of densities and the peak of the curve should be generally higher. We thus suggest that our model may yield the basis for further exploring of density-dependence between different species.
Our overall findings indicate that plant–plant interactions change predictably—but partially differently than suggested by the SGH—along stress gradients. The striking consistency between our quantitative model and experiments indicates that the findings are robust. Including density-dependence in models of facilitation will undoubtedly promote to predict more precisely how vegetation could respond to environmental change. Furthermore, the findings could provide insights into species coexistence hypotheses, which mainly focus on competition and often assume that the overall effect of one species on another is linearly and negatively related to its density19,20. However, we show that unimodal relationships should occur and that these depend on stress. Our findings could also be helpful for ecological restoration and crop production29. Because of the universal nonoptimal habitat conditions and ongoing environmental change17,18, we advocate the inclusion of density as a factor mediating positive and negative plant interactions in experiments and models. Our study represents a robust contribution to such an approach and may help to reconcile competition and facilitation theory into a common framework.
Methods
Model
A detailed model description is provided in the Supplementary methods. Here, we only illustrate the core equations. In the model, an individual’s potential growth rate (without neighbors) is defined as:
| 1 |
where m is total mass, a is a species-specific constant, and M is the theoretically maximum mass. S is the stress intensity quantified as the proportional reduction in incoming energy. It ranges from 0 (no stress) to 1 (extreme stress and no resources).
The equation is based on Metabolic Scaling Theory52 and it was integrated with the zone-of-influence (ZOI) model to include interactions53. In the spatially-explicit ZOI model, each plant is modeled as a circle and its size (A, unit: area) denotes potentially available resources and is allometrically related to the biomass, as A = C0m3/4, where C0 is a constant (see Supplementary methods). Individuals interact with each other where their ZOIs overlap. Therefore, the realized growth rate is as follows:
| 2 |
where r indicates the intrinsic growth rate (in mass area–1 time–1) while Ic and If are competition and facilitation indexes, respectively. Ic represents the proportion of actual resources available for an individual under competition:
| 3 |
where Ac represents the actual resources a plant can obtain. It is calculated as the sum of the nonoverlapping area and the effective that can be obtained within overlapping zones. The division of overlapping areas is determined by the parameter p, i.e., the mode of competition53. It ranges from 0 (complete symmetry) to ∞ (complete asymmetry). In harsh conditions, the size of ZOI also reflects the quantity of perceived stress and stress mitigation occurs in overlapping areas. Similarly, If is the proportion of realized stress for an individual experiencing facilitation:
| 4 |
where Af is a quantitative measure for stress reduction by neighbors. Likewise, the calculation is based on how individuals share overlapping ZOIs (stress), which is determined by the parameter q, i.e., the mode of facilitation51. It also ranges from 0 (complete symmetric facilitation) to ∞ (complete asymmetric facilitation). See also Supplementary methods for detailed calculation process about Ic and If.
Compared to previous models, a main improvement is the explicit consideration of the impact of the size of the beneficiary on its facilitative response. Previous studies have used the term S/(1 + Af) for effects of stress and facilitation on plant performance31,40,49–51. For a given stress level, this term is determined only by Af, i.e., positive effects of benefactors. However, this term is not realistic because it cannot reflect differential sensitivity of beneficiaries with different size to stress amelioration (facilitative response, see Fig. 5 for example). Thus, the influence of facilitation was identical: S/(1 + Afa) = S/(1 + Afb), as long as stress alleviation by benefactors was the same (Afa = Afb), although the smaller plant should experience stronger facilitation. A fundamental problem arising from that approach is that the unit of Af (area–1) is not balanced in the term S/(1+ Af), and units on both sides of the growth equation are not identical. Namely, the units are mass time–1 on the left side but mass time–1 area–140,51 or even more complicated on the right side31,50. Here, we included facilitative response via the term SIf = S(1 − Af/A), which is both more realistic and mathematically correct (equal units: mass time−1).
Fig. 5. Schematic illustration of stress amelioration for plants with different size in a zone-of-influence (ZOI) model.
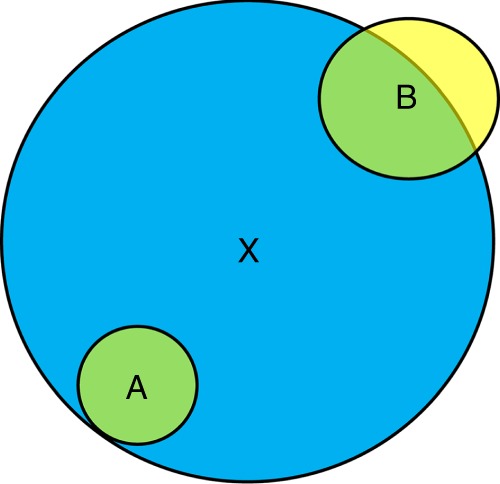
A and B (yellow ZOIs) are two conspecific beneficiary plants and they are facilitated by a benefactor X (blue ZOI). The stress intensity (e.g., salinity) per unit area is homogeneous across space. The area of overlap of ZOIs (green) is the same for both beneficiary plants (Afa = Afb). However, plant A fully benefits from the presence of X while B benefits only partly. Thus, beneficiary A will respond more positively to habitat amelioration than beneficiary B, i.e., the facilitative response is size dependent.
The simulations were done in Netlogo (version 6.0.1)54 using a grid of homogeneous patches (200 × 200) in a continuous two-dimensional space53 with a wrap-around approach to avoid edge effects55. Data were collected every ten time steps and results presented after 50 steps. Plants experienced a density gradient with seven levels from 2 to 8 (in scale), which also encompassed the density range of our experiment, Namely, in the control (without stress), the competition intensity at the minimum and maximum density level in simulations should be lower and higher than that in the experiment, respectively. Following previous studies22,31, density refers to initial density and not surviving density. We explored a wide range of stress levels and for ease of visualization, present here four of them: no (0), low (0.45), high (0.75), and extreme (0.85), respectively. Other stress levels yielded the same qualitative results (Supplementary Fig. 1). We also evaluated potential effects of spatial distribution (random, regular, aggregated) and different modes of competition and facilitation, but these did not change the qualitative findings either (Supplementary Fig. 5). In the following, we thus mainly present results for the scenario most similar to the intraspecific experiment, i.e., random distribution, symmetric competition (p = 1) and symmetric facilitation (q = 1). Each simulation was repeated five times. Considering that plant–plant interactions may change over time due to individual growth, we also ran additional simulations. Thus, in each time step, we collected data of the mean biomass under relatively high stress where strong facilitation occurred, i.e., S = 0.75 and 0.85.
The RII was used to quantify strength and direction of net plant–plant interactions56:
| 5 |
where PW and PS are the performance of plants with and without neighbors, respectively. RII ranges from −1 to 1 with negative values indicating competition and positive values net facilitative interactions. To estimate biomass of individuals without neighbors (PS), we calculated the growth rate as follows:
| 6 |
Experiment
The experiment was set up in a greenhouse at Tübingen University between early October and mid December 2015 (light intensities: 130–150 µmol m−2 s−1 and 16 h day−1; temperatures: 15 °C (night) and 20 °C (day)). We selected the model plant Arabidopsis thaliana as study species because it is annual and fast-growing, densities can be easily manipulated, and standardized seed material enables to conduct experiments with essentially similar plants. Moreover, previous research has revealed strong intraspecific facilitation under salt stress for this species34 such that the general system was well established. Seeds of A. thaliana (popular Columbia wildtypes) were obtained from Nottingham Arabidopsis Stock Centre and inbred lines raised for one generation to amplify seed numbers.
Seeds were sterilized in 70% ethanol for 2 min and stratified at 4 °C for 5 days57. They were then transferred to pots (10 × 10 × 10 cm) with standard potting soil (Einheitserde Classic, Gebr. Patzer Company). Seeds were sown to obtain a density gradient, i.e., 1 (without interactions), 2, 3, 5, 7, 10, 15, 20 seedlings per pot, respectively. Considering the small pots, 20 individuals per pot was actually very dense (i.e., 2000 plants per square meter). Approximately twice the number was sown in each pot for making sure that the desired seedling numbers can be obtained even under incomplete germination. We further prepared 40 additional pots with 10 seeds each as backup. Three days after germination, randomly selected surplus plants were removed. 60 spare individuals were transplanted to pots with insufficient germination. Plants growing with fewer neighbors may suffer more from stress58, and we observed high mortality of single plants under high salinity in our pilot study. We therefore set more replicates for low densities to obtain enough individuals for the analyses, i.e., 50 for density 1, 30 for 2 and 3, and 20 for each of the higher densities, respectively.
We applied the following four treatments: 0 (no salt), 50 (low), 100 (high), and 150 mM NaCl (extreme) solution. They were selected based on a previous study34 and they were meant to capture the entire possible range from no stress to extreme stress i.e., 80% limitation in performance such as growth and/or survival for plants without neighbors35. Naturally observed stress levels in saline habitats are well within this range, e.g., highly saline conditions may limit growth by up to about 70–75%29,43. Salt was first applied eight days after germination and plants were watered every 5–7 days depending on soil water conditions. Each pot received the same amount of liquid at each irrigation event, and the amount was successively increased from 50 ml to 150 ml as plants grew. The location of pots assigned to different treatments was fully randomized. At the end of the experiment, we counted the number of survivors in each pot and determined the number of siliques and above-ground biomass (dried at 70 °C for 24 h) per survivor.
RIIs were also calculated according to Eq. (5). Since the experiment was an intraspecific setting, we calculated PW and PS as the mean individual performance per pot without and with interactions, respectively31.
Statistical analysis
We applied Bayesian inference and fitted different models to the dataset of RIIs of simulations and the experiment, respectively (sample size in each treatment were provided in Source data). To compare our own conceptual model with existing theory, four models (Table 1) were fitted: (1) only stress as explanatory variable, which corresponds to classical facilitation models, i.e., SGH, a unidirectional change of RII along a stress gradient; (2) only density, i.e., classical competition models; (3) both density and stress (without the interaction term), i.e., the model that the RII–density relationship simply moves upward with increasing stress); (4) density, stress and their interactions (our own model).
When calculating the joint posterior distribution and uncertainty intervals of each model parameter, we selected uninformative Gaussian priors because there were few previous studies considering how density-dependence of facilitation could change along stress gradients59. In the calculation, Markov chain Monte Carlo (MCMC) were applied with 20,000 iterations60. After the estimation of posterior, we further investigated the convergence, i.e., whether MCMC chains converged to the posterior . The Watanabe–Akaike information criterion (WAIC) was used for comparing these Bayesian models and the model with the smallest WAIC value was selected to be the best model61. The uncertainty in model selection was also evaluated, by using weighted averages of all the models59,62. All Bayesian statistics and data calculations were performed in R (version 3.6.1)63 using the package brms60, which is based on the probabilistic programming language Stan64.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We are grateful to Dr. Xiangzi Zheng for his help in the greenhouse. We also thank Dr. Yue Lin for sharing his codes. R.Z. was supported by the China Scholarship Council (CSC).
Author contributions
R.Z. and K.T. conceived the ideas and wrote the manuscript; R.Z. performed the modeling work and experiments and analyzed the data.
Data availability
The source data underlying Figs. 2a, b, 3a, b, and 4a–h, Supplementary Figs. 1, 2a–d, 3a–d, 4a–h, 5a, b, and 6a–d, Table 1 and Supplementary Table 1 are provided as a Source Data file. The file is available at GitHub: https://github.com/Halili-z/density-dependence.
Code availability
Codes are deposited into a public repository (GitHub): https://github.com/Halili-z/density-dependence.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Christian Damgaard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-16286-6.
References
- 1.Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977;111:1169–1194. [Google Scholar]
- 2.Zhang J, Huang S, He F. Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. Proc. Natl Acad. Sci. USA. 2015;112:4009. doi: 10.1073/pnas.1420844112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dormann CF, et al. Biotic interactions in species distribution modelling: 10 questions to guide interpretation and avoid false conclusions. Glob. Ecol. Biogeogr. 2018;00:1–13. [Google Scholar]
- 4.Ettinger A, HilleRisLambers J. Competition and facilitation may lead to asymmetric range shift dynamics with climate change. Glob. Change Biol. 2017;23:3921–3933. doi: 10.1111/gcb.13649. [DOI] [PubMed] [Google Scholar]
- 5.Cavieres LA, et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol. Lett. 2014;17:193–202. doi: 10.1111/ele.12217. [DOI] [PubMed] [Google Scholar]
- 6.Bertness MD, Callaway RM. Positive interactions in communities. Trends Ecol. Evol. 1994;9:191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 7.Callaway RM, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- 8.He Q, Bertness MD, Altieri AH. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 2013;16:695–706. doi: 10.1111/ele.12080. [DOI] [PubMed] [Google Scholar]
- 9.Le Bagousse-Pinguet Y, Forey E, Touzard B, Michalet R. Disentangling the effects of water and nutrients for studying the outcome of plant interactions in sand dune ecosystems. J. Veg. Sci. 2013;24:375–383. [Google Scholar]
- 10.Maestre FT, Cortina J. Do positive interactions increase with abiotic stress?—A test from a semi-arid steppe. Biol. Lett. 2004;271:S331–S333. doi: 10.1098/rsbl.2004.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalet R, et al. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 2006;9:767–773. doi: 10.1111/j.1461-0248.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- 12.Tielbörger K, Kadmon R. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology. 2000;81:1544–1553. [Google Scholar]
- 13.Koyama A, Tsuyuzaki S. Facilitation by tussock-forming species on seedling establishment collapses in an extreme drought year in a post-mined Sphagnum peatland. J. Veg. Sci. 2013;24:473–483. [Google Scholar]
- 14.Baraza E, Zamora R, Hódar A. J. Conditional outcomes in plant–herbivore interactions: neighbours matter. Oikos. 2006;113:148–156. [Google Scholar]
- 15.Forey E, Touzard B, Michalet R. Does disturbance drive the collapse of biotic interactions at the severe end of a diversity-biomass gradient? Plant Ecol. 2010;206:287–295. [Google Scholar]
- 16.Scheiter S, Langan L, Higgins SI. Next-generation dynamic global vegetation models: learning from community ecology. N. Phytol. 2013;198:957–969. doi: 10.1111/nph.12210. [DOI] [PubMed] [Google Scholar]
- 17.Soliveres S, Smit C, Maestre FT. Moving forward on facilitation research: response to changing environments and effects on the diversity, functioning and evolution of plant communities. Biol. Rev. 2015;90:297–313. doi: 10.1111/brv.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooker RW. Plant–plant interactions and environmental change. N. Phytol. 2006;171:271–284. doi: 10.1111/j.1469-8137.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 19.Adler PB, et al. Competition and coexistence in plant communities: intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018;21:1919–1329. doi: 10.1111/ele.13098. [DOI] [PubMed] [Google Scholar]
- 20.Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. [Google Scholar]
- 21.Levine JM, Bascompte J, Adler PB, Allesina S. Beyond pairwise mechanisms of species coexistence in complex communities. Nature. 2017;546:56–64. doi: 10.1038/nature22898. [DOI] [PubMed] [Google Scholar]
- 22.Weiner J, Freckleton RP. Constant final yield. Annu. Rev. Ecol., Evol. Syst. 2010;41:173–192. [Google Scholar]
- 23.Yoda K, Kira T, Ogawa H, Hozumi K. Self-thinning in overcrowded pure stands under cultivated and natural conditions. J. Biol. Osaka City Univ. 1963;14:107–129. [Google Scholar]
- 24.Travis JMJ, Brooker RW, Dytham C. The interplay of positive and negative species interactions across an environmental gradient: insights from an individual-based simulation model. Biol. Lett. 2005;1:5–8. doi: 10.1098/rsbl.2004.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maestre F, Callaway R, Valladares F, Lortie C. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009;97:199–205. [Google Scholar]
- 26.Malkinson D, Tielbörger K. What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos. 2010;119:1546–1552. [Google Scholar]
- 27.Callaway RM, Walker LR. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- 28.Bishop MJ, Byers JE, Marcek BJ, Gribben PE. Density-dependent facilitation cascades determine epifaunal community structure in temperate Australian mangroves. Ecology. 2012;93:1388–1401. doi: 10.1890/10-2296.1. [DOI] [PubMed] [Google Scholar]
- 29.Silliman BR, et al. Facilitation shifts paradigms and can amplify coastal restoration efforts. Proc. Natl Acad. Sci. USA. 2015;112:14295. doi: 10.1073/pnas.1515297112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callaway RM. Positive interactions among plants. Bot. Rev. 1995;61:306–349. [Google Scholar]
- 31.Chu C-J, et al. Balance between facilitation and resource competition determines biomass–density relationships in plant populations. Ecol. Lett. 2008;11:1189–1197. doi: 10.1111/j.1461-0248.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 32.Xi X, Mu J, Peng Y, Eisenhauer N, Sun S. Capitulum density-dependent effects generate peak seed yield at an intermediate density of a Tibetan lotus. J. Plant Ecol. 2016;9:100–106. [Google Scholar]
- 33.Dickie IA, Schnitzer SA, Reich PB, Hobbie SE. Spatially disjunct effects of co-occurring competition and facilitation. Ecol. Lett. 2005;8:1191–1200. doi: 10.1111/j.1461-0248.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Tielbörger K. Facilitation from an intraspecific perspective—stress tolerance determines facilitative effect and response in plants. N. Phytol. 2019;221:2203–2212. doi: 10.1111/nph.15528. [DOI] [PubMed] [Google Scholar]
- 35.He Q, Bertness MD. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology. 2014;95:1437–1443. doi: 10.1890/13-2226.1. [DOI] [PubMed] [Google Scholar]
- 36.Michalet R, Schöb C, Lortie CJ, Brooker RW, Callaway RM. Partitioning net interactions among plants along altitudinal gradients to study community responses to climate change. Funct. Ecol. 2014;28:75–86. [Google Scholar]
- 37.Liancourt P, Callaway RM, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005;86:1611–1618. [Google Scholar]
- 38.Michalet R, Le Bagousse-Pinguet Y, Maalouf J-P, Lortie CJ. Two alternatives to the stress-gradient hypothesis at the edge of life: the collapse of facilitation and the switch from facilitation to competition. J. Veg. Sci. 2014;25:609–613. [Google Scholar]
- 39.Schöb C, Armas C, Guler M, Prieto I, Pugnaire FI. Variability in functional traits mediates plant interactions along stress gradients. J. Ecol. 2013;101:753–762. [Google Scholar]
- 40.Lin Y, Berger U, Yue M, Grimm V. Asymmetric facilitation can reduce size inequality in plant populations resulting in delayed density-dependent mortality. Oikos. 2016;125:1153–1161. [Google Scholar]
- 41.Sthultz CM, Gehring CA, Whitham TG. Shifts from competition to facilitation between a foundation tree and a pioneer shrub across spatial and temporal scales in a semiarid woodland. N. Phytol. 2007;173:135–145. doi: 10.1111/j.1469-8137.2006.01915.x. [DOI] [PubMed] [Google Scholar]
- 42.le Roux PC, Shaw JD, Chown SL. Ontogenetic shifts in plant interactions vary with environmental severity and affect population structure. N. Phytol. 2013;200:241–250. doi: 10.1111/nph.12349. [DOI] [PubMed] [Google Scholar]
- 43.Bertness MD, Hacker SD. Physical stress and positive associations among marsh plants. Am. Nat. 1994;144:363–372. [Google Scholar]
- 44.Watkinson AR. Density-dependence in single-species populations of plants. J. Theor. Biol. 1980;83:345–357. [Google Scholar]
- 45.Brooker RW, et al. Facilitation in plant communities: the past, the present, and the future. J. Ecol. 2008;96:18–34. [Google Scholar]
- 46.Enquist BJ, Brown J, West G. Allometric scaling of plant energetics and population density. Nature. 1998;395:163–165. [Google Scholar]
- 47.Goldenheim W, Irving A, Bertness M. Switching from negative to positive density-dependence among populations of a cobble beach plant. Oecologia. 2008;158:473–483. doi: 10.1007/s00442-008-1157-0. [DOI] [PubMed] [Google Scholar]
- 48.Bos AR, van Katwijk MM. Planting density, hydrodynamic exposure and mussel beds affect survival of transplanted intertidal eelgrass. Mar. Ecol.-Prog. Ser. 2007;336:121–129. [Google Scholar]
- 49.Jia X, Dai X-F, Shen Z-X, Zhang J-Y, Wang G-X. Facilitation can maintain clustered spatial pattern of plant populations during density-dependent mortality: insights from a zone-of-influence model. Oikos. 2011;120:472–480. [Google Scholar]
- 50.Chu C-J, et al. Positive interactions can increase size inequality in plant populations. J. Ecol. 2009;97:1401–1407. [Google Scholar]
- 51.Lin Y, Berger U, Grimm V, Ji Q-R. Differences between symmetric and asymmetric facilitation matter: exploring the interplay between modes of positive and negative plant interactions. J. Ecol. 2012;100:1482–1491. [Google Scholar]
- 52.West GB, Brown JH, Enquist BJ. A general model for ontogenetic growth. Nature. 2001;413:628–631. doi: 10.1038/35098076. [DOI] [PubMed] [Google Scholar]
- 53.Weiner J, Stoll P, Muller‐Landau H, Jasentuliyana A. The effects of density, spatial pattern, and competitive symmetry on size variation in simulated plant populations. Am. Nat. 2001;158:438–450. doi: 10.1086/321988. [DOI] [PubMed] [Google Scholar]
- 54.Wilensky, U. Netlogo http://ccl.northwestern.edu/netlogo/. (Center for Connected Learning and Computer-Based Modeling, Northwestern University. Evanston, IL, 1999).
- 55.Grimm, V. & Railsback, S. F. Individual-Based Modeling and Ecology (Princeton University Press, 2005).
- 56.Armas C, Ordiales R, Pugnaire F. Measuring plant interactions: a new comparative index. Ecology. 2004;85:2682–2686. [Google Scholar]
- 57.Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family of nad-dependent deacetylases by phenotypic screening. J. Biol. Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- 58.Fajardo A, McIntire EJB. Under strong niche overlap conspecifics do not compete but help each other to survive: facilitation at the intraspecific level. J. Ecol. 2011;99:642–650. [Google Scholar]
- 59.Ellison AM. Bayesian inference in ecology. Ecol. Lett. 2004;7:509–520. [Google Scholar]
- 60.Bürkner P-C. BRMS: an R package for bayesian multilevel models using stan. J. Stat. Softw. 2017 doi: 10.18637/jss.v080.i01. [DOI] [Google Scholar]
- 61.Hooten MB, Hobbs NT. A guide to Bayesian model selection for ecologists. Ecol. Monogr. 2015;85:3–28. [Google Scholar]
- 62.Dormann CF, et al. Model averaging in ecology: a review of Bayesian, information-theoretic, and tactical approaches for predictive inference. Ecol. Monogr. 2018;88:485–504. [Google Scholar]
- 63.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2016).
- 64.Carpenter B, et al. Stan: a probabilistic programming language. J. Stat. Softw. 2017 doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data underlying Figs. 2a, b, 3a, b, and 4a–h, Supplementary Figs. 1, 2a–d, 3a–d, 4a–h, 5a, b, and 6a–d, Table 1 and Supplementary Table 1 are provided as a Source Data file. The file is available at GitHub: https://github.com/Halili-z/density-dependence.
Codes are deposited into a public repository (GitHub): https://github.com/Halili-z/density-dependence.