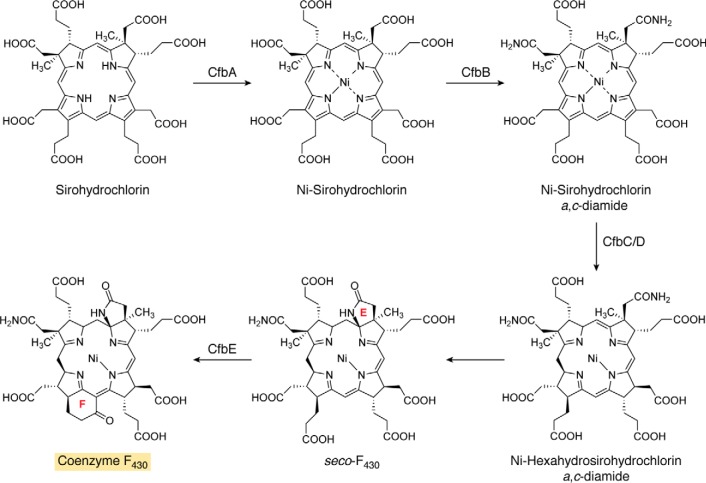
Figure 4.
The transformation of sirohydrochlorin into coenzyme F430. The steps involved in the biosynthesis of F430 from sirohydrochlorin are outlined. Initially, sirohydrochlorin is chelated with nickel by the enzyme CfbA to give nickel sirohydrochlorin. Next, the two acetic acid side chains on rings A and B, the a and c side chains, are amidated in a reaction catalyzed by CfbB that also requires glutamine and ATP as substrates. This generates nickel sirohydrochlorin a,c-diamide, which acts as the substrate for the reductase system that is catalyzed by CfbC and -D. The reductase removes three double bonds from the macrocycle, which also spontaneously results in the formation of the lactam ring E, thereby generating seco-F430. The final step, mediated by CfbE, results in the formation of the cyclic hexanone ring F in another ATP-requiring process. The shaded box for coenzyme F430 coordinates with other pathway figures and the summary depiction in Fig. 14.