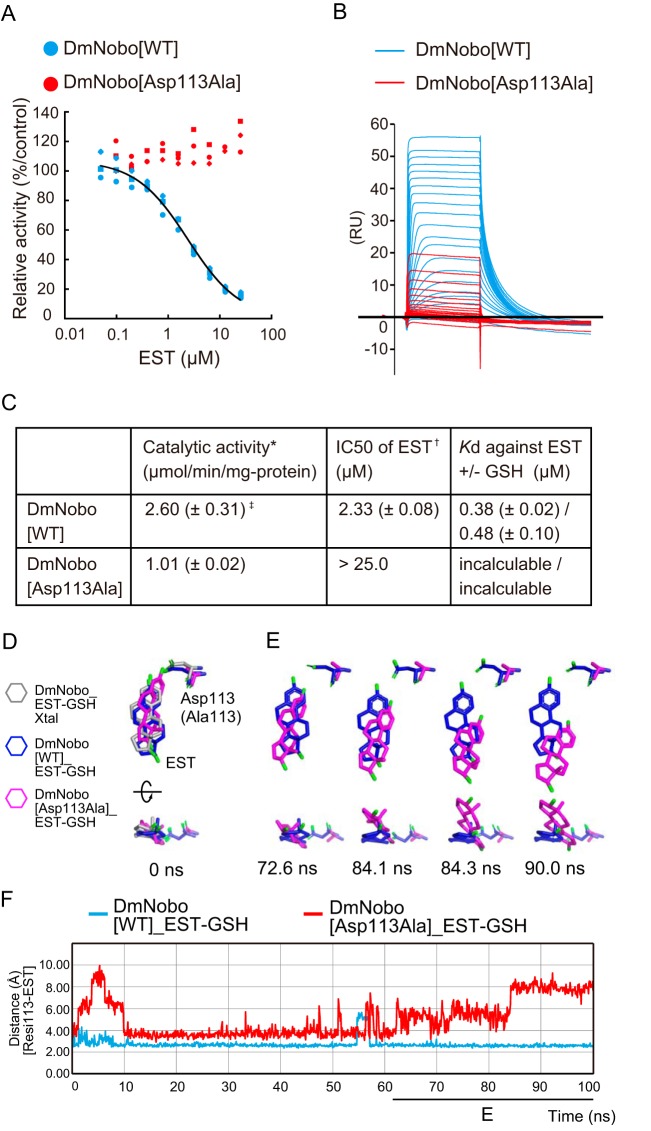
Figure 4.
Asp-113 is essential for DmNobo binding to EST. A, EST-dependent inhibition of the GSH conjugation activity of DmNobo WT (cyan) and DmNobo D113A (red). 3,4-DNADCF was used as an artificial fluorescent substrate. In each case, the relative activity is defined as the ratio of activity, when compared with DmNobo WT without EST. All of the data points in triplicate assays are shown. B, sensorgrams of surface plasmon resonance analysis of DmNobo proteins with EST. DmNobo WT or DmNobo D113A was immobilized to a sensor chip, and solutions containing a series of EST concentrations were applied in presence of 1 mm GSH. C, kinetic parameters of DmNobo proteins. Catalytic activity (*) and IC50 of EST (†) indicate 3,4-DNADCF-specific GSH conjugation activity and the IC50 of EST against 3,4-DNADCF–specific GSH conjugation activity, respectively. Values in parentheses indicate S.D. from triplicate assays (‡). D–F, in silico evaluation of the contribution of Asp-113 to the interaction between DmNobo and EST. MD simulations of the DmNobo WT or DmNobo D113A complex with EST and GSH in a TIP3P-water model were carried out at 300 K for 100 ns. These simulations were performed in triplicate. D, MD models at 0 ns of DmNobo with EST and GSH (blue), DmNobo D113A with EST and GSH (magenta), and the crystal structure of DmNobo_EST-GSH (EST-GSH_Xtal, gray). The upper models are shown from above the EST ligand, and the lower models are rotated 90° from the upper models. Hydrogen atoms are not shown. E, MD models of DmNobo WT_EST-GSH and DmNobo D113A_EST-GSH from 72.6 ns to 90.0 ns. F, distance between Oδ of Asp-113 of DmNobo WT or Cβ of DmNobo D113A and the O3 atom of EST at each frame.