Figure 2.
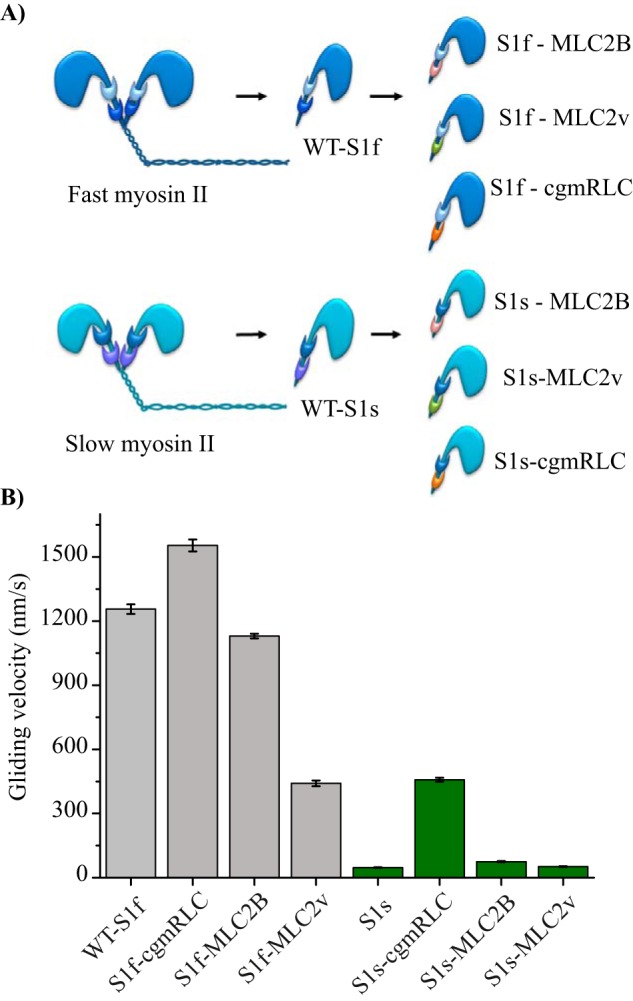
Reconstitution of chimeric motors. A, scheme illustrates in vitro reconstituted motors with different combinations of RLCs with fast (dark blue) and slow myosin II (sky blue) heavy chains. Papain digestion of full-length myosin II generated single-headed myosin S1. Regulatory light chains are color-coded to indicate the exchange: MLC2B (pink), MLC2v (green), and cgmRLC (orange). B, in vitro motility assay using native myosin S1 (WT-S1f and WT-S1s) or with chimeric motors. Motors were immobilized on a nitrocellulose-coated surface. Speed of movement was measured at saturating ATP concentration of 2 mm at room temperature (22 °C). Bar diagrams show the reduction in mean velocity from 1.2 ± 0.19 for WT-S1f to 0.44 ± 0.13 μm/s for S1f-MLC2v (N = 72 and 100 actin filaments, respectively) and the increase in mean velocity for WT-S1s from 0.047 ± 0.05 to 0.40 ± 0.05 μm/s for S1s-cgmRLC (N = 82 and 166 filaments, respectively). The motility experiments were performed with at least three different preparations of myosin motors and chimeras and were highly reproducible. For S1f-cgmRLC, N = 70; for S1f-MLC2B, N = 110; for S1s-MLC2v, N = 56; and for S1s-MLC2B, N = 55. Error bars, S.E. Statistical significance was calculated using unpaired t test for the following pairs of motors: WT-S1f and S1f-cgmRLC, p < 0.0001; WT-S1f and S1f-MLC2v, p < 0.0001; WT-S1s and S1s-cgmRLC, p < 0.0001; WT-S1s and S1s-MLC2B, p < 0.0001.
