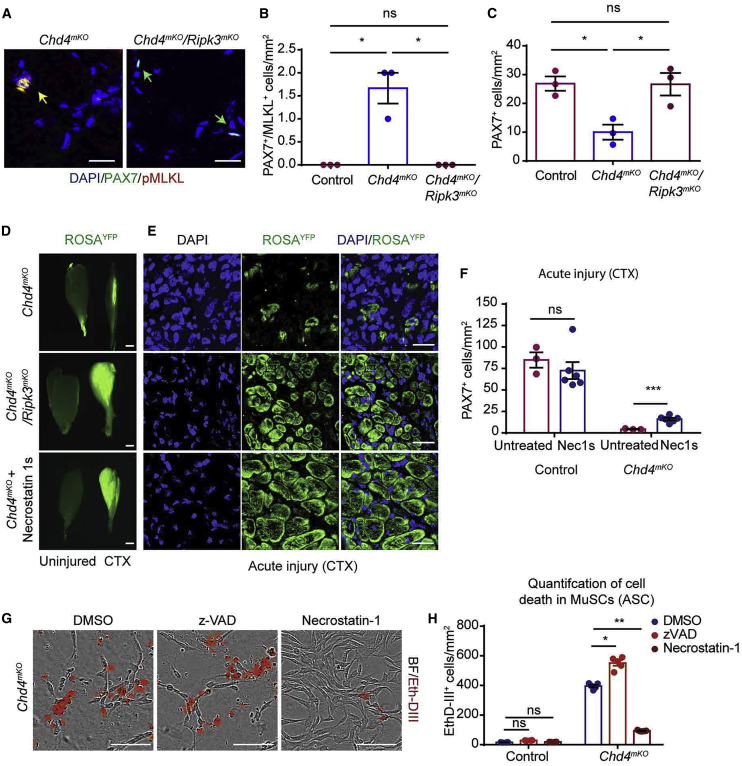
Figure 6.
Necrostatin Restores Proliferation of Chd4mKO MuSCs and Improves Skeletal Muscle Regeneration in Chd4mKO Mice
(A) Immunofluorescence staining for PAX7/pMLKL on TA muscle sections from Chd4mKO and Chd4mKO/Ripk3mKO mice. Scale bar, 25 μm.
(B and C) Quantification of PAX7+/pMLKL+ MuSCs (B) undergoing necroptosis and PAX7+ MuSCs (C) in control, Chd4mKO, and Chd4mKO/Ripk3mKO muscles (n = 3 for each group).
(D) Fluorescence images of uninjured (left) and injured (right) TA muscles from Chd4mKO/ROSA26YFP, Chd4mKO/Ripk3mKO/ROSA26YFP, and Chd4mKO/ROSA26YFP mice treated with Nec-1s 2 weeks after CTX-induced muscle injury (n = 3–5 for each group). Scale bar, 100 μm.
(E) Cross sections of injured TA muscles as in (D) indicating increased formation of YFP+ myofibers after Ripk3 inactivation. Scale bar, 25 μm.
(F) Quantification of MuSCs in Chd4mKO/ROSA26YFP TA muscles with and without Nec-1s treatment after CTX-induced muscle injury (n = 3–6 for each group.
(G) EthD-III incorporating Chd4mKO MuSCs after treatment with z-VAD, necrostatin-1, or DMSO (n = 3 for each group); scale bar, 100 μm.
(H) Quantification of EthD-III incorporating Chd4mKO MuSCs after 100 h of in vitro culture in a field of 1 mm2 following treatment with z-VAD, necrostatin-1, or DMSO (n = 3–6 for each group. Statisical analysis: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.005, two-way ANOVA followed by Bonferroni post-test with alpha = 5%).
All analyses indicated across the experiments were biological replicates unless otherwise stated.