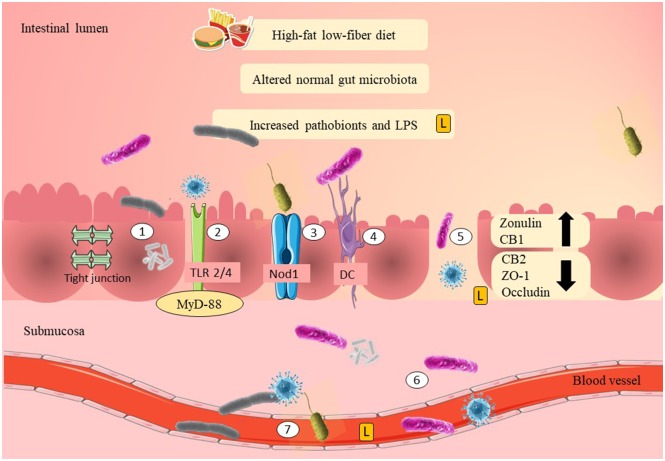
Figure 1.
Possible mechanisms of adherence of pathobionts and translocation across the epithelial layer of the gut in GDM. High-fat/low-fiber diet intake might have modulated the normal gut microbiota composition and increased the Gram-negative pathobionts. Elevation of Gram-negative pathobionts might have increased the LPS levels. There are several mechanisms as to how pathobionts and LPS are able to move across the epithelial layer of the gut. The first mechanism is by adherence to the mucosal layer. LPS and pathobionts might have crossed the epithelial layer of the gut through TLR 2/4 activation and is associated with the recruitment of MyD-88. LPS and pathobionts might have crossed the epithelial layer of the gut by binding to Nod1. DCs might have translocated pathobionts by phagocytosis and co-localization of the pathobionts from the intestinal lumen to the systemic circulation. Thin mucosal layer, depletion of tight junction proteins (ZO-1 and occludin), reduction of CB2, and elevation of CB1 may have increased the gut epithelial permeability (i.e., “leaky gut”). “Leaky gut” might have allowed translocation of LPS and pathobionts across the epithelial layer of the gut. LPS and pathobionts might have translocated from the intestinal lumen to the lamina propria and submucosa. LPS and pathobionts might have translocated from the submucosa to the systemic circulation and traveled to the peripheral tissues, including adipose, liver, and skeletal muscle. LPS, lipopolysaccharide; L, lipopolysaccharide; TLR2/4, toll-like receptor 2/4; Nod1, nod-like receptor 1; DC, dendritic cell; CB1/2, cannabinoid receptor 1/2; ZO-1, zona occludens 1; MyD-88, adapter proteins, myeloid differentiation factor.