Abstract
In the philosophy of medicine, great attention has been paid to defining disease, yet less attention has been paid to the classification of clinical conditions. These include conditions that look like diseases but are not; conditions that are diseases but that (currently) have no diagnostic criteria; and other types, including those relating to risk for disease. I present a typology of clinical conditions by examining factors important for characterizing clinical conditions. By attending to the types of clinical conditions possible on the basis of these key factors (symptomaticity, dysfunction, and the meeting of diagnostic criteria), I draw attention to how diseases and other clinical conditions as currently classified can be better categorized, highlighting the issues pertaining to certain typology categories. Through detailed analysis of a wide variety of clinical examples, including Alzheimer disease as a test case, I show how nosology, research, and decisions about diagnostic criteria should include normative as well as naturalistically describable factors.
Keywords: Alzheimer disease, Asymptomatic, Biostatistical theory, Classification, Diagnosis, Disease, Nosology, Type 2 diabetes
Highlights
-
•
A typology comprising 8 categories encompassing all clinical conditions is described.
-
•
Symptomaticity, dysfunction, & meeting of diagnostic criteria create the categories.
-
•
The typology can help think through implications of different classifications.
When citing this paper, please use the full journal title Studies in History and Philosophy of Biological and Biomedical Sciences.
1. Introduction
Clinical medicine is replete with examples of diseases where there is a disparity between the diagnostic criteria used to ascertain the presence of the disease and the pathophysiological dysfunction of the disease. Clinicians also study and treat a number of non-diseases, such as pregnancy, as well as conditions straddling the line between disease and risk for disease (such as the early stages of atherosclerosis). As Christopher Boorse wrote in 1977 (p. 564–565), “The distinction between normal variation and underlying disease is one of the most important features of medical theory, though in practice it is often hard to draw because so much clinical evidence is gross output.” Given that most clinical evidence is indeed gross output (blood glucose levels, for example), whereby laboratory tests at most only offer a proxy for or rough indication of the dysfunction present (e.g., defects in pancreatic beta cells, adipose tissue, the incretin system), ascertaining the presence of disease is not always as straightforward as it may seem. This is especially true for asymptomatic conditions, but also for conditions in which the dysfunction is mild or difficult to discern. Although there are multiple nosologies of disease, there is less discussion in the philosophical or medical literature on the classification of clinical conditions (with some exceptions, such as Kline, 1986; Engelhardt, 1996, ch. 5; Caplan, McCartney, & Sisti, 2004; Schwartz, 2008, 2014a; Doust, Walker, & Rogers, 2017; and Hofmann, 2016, 2017). Clinical conditions include conditions that look like diseases but are not; conditions that are diseases but that (currently) have no diagnostic criteria; and other types, including those relating to risk for disease (Tresker, 2020). This paper is meant to fill that gap by examining factors important for characterizing clinical conditions, to arrive at a typology of clinical conditions. Through analysis of a wide variety of detailed clinical examples, I show how nosology, research, and decisions about diagnostic criteria should include normative as well as naturalistically describable factors, even when relying on a naturalist conception of disease.
First some definitions are in order. My conception of disease is specified by Boorse's biostatistical theory (BST; Boorse, 2014, p. 684). The BST has seen its fair share of criticism, with many authors finding the naturalist conception of disease to be untenable. The typology in part serves the purpose of justifying using “disease” in this particular way, and therefore implicitly underscores the BST's usefulness. By the BST, biological dysfunction is necessary for a condition to be a disease. Following from the other requirements of the BST (discussed in Boorse, 2014), one way to characterize disease is as the pathophysiological defects constituting a statistical subnormality of physiological function.
As is explained in detail later, the typology is formed from three factors: symptomaticity, dysfunction, and the meeting of diagnostic criteria. The intersection of these factors creates the typology's eight categories. These categories clarify relationships among clinical conditions (and show how they extend beyond a narrow focus on disease), and provide a framework for understanding diagnostic criteria, how they change, and why they should change, such as depending upon what (if any) symptoms a person exhibits at a given time. Unless otherwise mentioned, by diagnostic criteria I mean current criteria. Typically these involve the presence of symptoms and signs (such as results of laboratory tests, imaging, genetic analysis). However, for many conditions, such as cluster headache, mostly or only symptoms constitute official diagnostic criteria (e.g., those promulgated by medical society guidelines for use in clinical practice). This highlights the fact that for many conditions the existence of pathophysiology is assumed on the basis of the presence of symptoms. Yet recent calls for revisions in Alzheimer disease (AD) and Huntington disease diagnostic criteria advocate inclusion of AD and Huntington disease biomarkers (Jack et al., 2018; Reilmann, Leavitt, & Ross, 2014). This underscores the notion that there is not always a close correspondence between AD and Huntington disease clinical symptoms and their respective pathophysiological signs. Indeed, pathological changes can be present in the brains of people with AD years before the manifestation of symptoms (Jack et al., 2011, p. 258), whereas people with Huntington disease possess the mutant gene from birth and often undergo a long presymptomatic phase (Reilmann et al., 2014). How these and other conditions are defined (through diagnostic criteria) has profound implications for clinical practice, research, and society (regarding insurance, stigma, and other effects of being labeled with a clinical condition). A chief goal of this paper is to explain how normative considerations determine which typology categories a condition can be in and therefore whether biological dysfunction or symptoms are more important for defining a disease. The usefulness of the typology may lie in both identifying main distinguishing features of clinical conditions but also in using the distinctions to think through the implications of different classifications.
2. Factors important for a typology of clinical conditions
2.1. Dysfunction
Dysfunction as a necessary feature of disease has been extensively defended by Boorse and others (e.g., Schwartz, 2014b; Hausman, 2012, 2014), including those advocating so-called hybrid conceptions of disease (e.g., Stegenga, 2015; Wakefield, 2014), so I will not repeat these arguments here. Since diseases should be included in any typology of clinical conditions meant to reflect clinical practice and medical science, dysfunction accordingly must be a feature of that typology.
A diagnosed condition does not automatically mean dysfunction is present, and undiagnosed disease does not necessarily mean there is no pathology present. Improved diagnostic criteria could better capture the people who truly have the physiological dysfunction constitutive of a disease. However, as will be discussed later, normative considerations and not any intrinsic feature of the BST or the typology determine whether this should be done.
The disparity between the diagnostic criteria used to ascertain the presence of disease and the underlying disease-defining dysfunction is known to practicing clinicians and medical researchers. For example, in acute kidney injury (AKI) it is clear that the current diagnostic tools (such as serum creatinine concentrations) do not accurately reflect the underlying pathophysiology:
… serum creatinine and urine output are markers of excretory function only and do not provide any information about any other roles of the kidney, i.e. metabolic, endocrine, or immunological functions. They are also not kidney specific and need to be interpreted within the clinical context. Some patients fulfil the AKI definition but do not have AKI, and there are also patients with clear evidence of renal injury who do not meet the creatinine or urine criteria for AKI. (Ostermann & Joannidis, 2016, p. 1)
Indeed, the arbitrariness of the current cut-off of oliguria has been recognized in the field (Md Ralib, Pickering, Shaw, & Endre, 2013; Ostermann, 2014). Yet because kidney biopsy is risky and impractical, more specific and sensitive diagnostic tools are needed than those commonly used. AKI has multiple etiologies and proper diagnostic tools should distinguish these. As will be discussed below, similar situations exist for other diseases, including chronic ones. The typology can make sense of such situations, as well as nosological developments, and thus provides a conceptual framework for thinking about what may be readily apparent to medical professionals, but also what may be contested territory.
2.2. Symptomaticity
On the BST asymptomatic disease is still disease because there is statistically subnormal physiological function. This dysfunction just does not (yet) manifest in symptoms. An arguably crucial factor, besides dysfunction, for forming a typology of clinical conditions is thus symptomaticity. This is because not only is the presence of symptoms an important feature of clinical conditions but so is their absence. In some cases symptoms point to the presence of disease, or, when absent, indicate the absence of a medical condition or the possibility of clinically undetected disease. There are many diseases, such as COVID-19 infection, atherosclerosis, and Huntington disease, that do not produce symptoms during some stages, for example.
My use of “symptoms” accords with medical usage, and covers any indication of a clinical condition as perceived by the patient. Symptoms thus represent the subjective features of a patient's clinical condition (in contrast to signs, which are the objective features clinicians observe or measure, such as blood pressure). Symptoms need not be communicated to a clinician to be a symptom and need not represent an underlying disease; thus, dyspnea is still a symptom if not observed by a pulmonologist, and does not necessarily indicate or reflect a lung disease, especially if experienced after strenuous exercise. Stiffness on awakening could be a symptom of arthritis, or a consequence of a bad bed. Hunger and unwanted feelings could be symptoms, even though they generally are not: patients do not typically complain of hunger, although when they do a clinician might inquire if the hunger is ravenous, in which case it could indicate a clinical condition (e.g., bulimia). But it would be the odd parent who takes a child complaining of hunger to the doctor instead of giving them food. What makes a feeling a medically important symptom is a question of judgment, and in many cases a judgment informed by medical knowledge and clinical experience.
Clinicians and patients decide whether a “symptom” is medically relevant, and sometimes their views diverge. For example, a psychiatrist could view a person's feeling of guilt over a minor infraction as being excessive and indicative of a disorder, although the person could feel it to be normal; similarly, a patient could view their fatigue as excessive and indicative of disease even if landing in the normal range on exercise testing. Failure to be brilliant and lack of optimal happiness or flourishing are not symptoms on my usage because they are signs. A feeling of failing to be brilliant or not being optimally happy could be a symptom, however, but typically is not. One reason clinicians mostly do not recognize any unwanted feeling as a medically relevant symptom is because in most cases an unwanted feeling is not indicative of disease. It could simply represent normal variation or a reaction to a life event or circumstance and not reflect the presence of an underlying biological dysfunction or disease process. Yet on the typology of clinical conditions they are still potentially medically relevant symptoms. Even something as vague and amorphous as “not feeling well” is commonly encountered in doctors' offices. The account of symptoms presented here is meant to reflect the patient's perspective and to accord primacy to their feelings, whether ultimately medically relevant or not with respect to diagnosis or treatment.
Symptomatology is an important factor for a typology of clinical conditions because symptoms are typically the primary indicators of a clinical condition—potentially a disease—that compel a patient to visit their doctor. In fact, approximately one fifth of outpatient consultations are because of medically unexplained symptoms (Reid, Wessely, Crayford, & Hotopf, 2001), meaning a patient could end up leaving their doctor's office still with a symptom and possibly feeling ill, yet with no diagnosis. On the typology they would still have a clinical condition, category E or G.
2.3. Meeting of diagnostic criteria
Another important factor for a typology of clinical conditions is the meeting of diagnostic criteria. This is because this factor determines whether a person is considered to have a disease (even if there is not always a correspondence between whether the person meets the BST's criteria for having a disease). Diagnostic criteria comprise signs, symptoms, or both. They are how clinicians diagnose a condition, whether the criteria are implicit or explicitly stated in guidelines. In practice, diagnoses may proceed on the basis of criteria different from those listed in guidelines, such as a physician practicing when the American College of Rheumatology's 1990 criteria for the diagnosis of fibromyalgia were followed who might have diagnosed a patient with fibromyalgia even in the absence of tender points, despite the requirement for their presence. However, this is not a problem for the typology because criteria are still being used and these criteria necessarily involve certain symptoms or signs.
Diagnostic methods (like a gold standard, say biopsy) are not the same as diagnostic criteria. The latter may encapsulate the former. A test itself is not a diagnostic criterion because it is a means by which a diagnostic criterion can be assessed as being met or not, typically by a cut-off value or the presence or absence of a feature (e.g., cancerous cells). There are many diseases for which the pathology is too difficult to assess, so the diagnostic criteria for the disease consist solely of symptoms or behavioral signs. Meeting diagnostic criteria is how diseases are identified in clinical practice. In some cases this involves specific pathology, as identified through whatever tests are available that could indicate the key dysfunction is present. In other cases this might solely involve symptoms, which for some conditions, at least some of the time (e.g., AD), provide a strong indication the key biological dysfunction is present, whereas for other conditions (e.g., depression) sometimes only tenuously (if at all) link to pathology.
The fact that most of the conditions listed in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5; American Psychiatric Association, 2013) are based on behavioral criteria (i.e., polythetic symptom complexes) emphasizes the importance of symptoms to the diagnostic criteria of many conditions. It also shows how for some of the DSM-5 conditions biological dysfunction could be present, whereas for others absent, a point also made by Olbert and Gala (2015) on their way to arguing for the distinct role played by symptomatology in mental versus somatic disorders. The intriguing example they give involves the difficulty believing a person could have a mental disorder without that person experiencing that disorder's defining symptoms (e.g., depression diagnosed by biomarkers in a patient without any depression symptoms; Olbert & Gala, 2015, p. 215). As will be discussed for AD, any incredulity this might engender depends on the condition; AD, like depression, is listed in the DSM-5, but unlike depression has biomarkers available that can identify AD's neuropathological signature even in a patient not yet exhibiting dementia. This raises the possibility of asymptomatic diagnosis, something conceivable for a neurological condition but seemingly bizarre for a psychiatric one.
Many diseases go undiagnosed. The typology shows that there could be disease even if the most conclusive test is not performed; consider herpes sufferers before the Tzanck smear was available. A person with painful pustules around their mouth could have an acne or herpes simplex outbreak. Confirming herpes with the Tzanck smear does not mean the disease was not present prior to performing the test. Indeed, whether a test accurately identifies herpes simplex as the cause is a function of its sensitivity and specificity, which is why the Tzanck smear is now rarely used in some locales given that polymerase chain reaction tests are available.
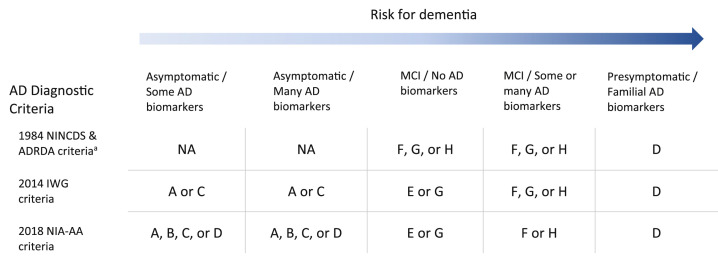
In a clinic a person is diagnosed with a disease or not. If yes, that diagnosis might not really represent disease (at least based on the presence of dysfunction), or if no then the person still has a clinical condition, which is partly known through the presence of certain symptoms. Some diagnostic criteria do a better job of identifying diseases than others. Diagnostic criteria for diseases are not static. They change over time, as when an expert group revises diagnostic criteria. This can be of profound significance for the conceptualization of a condition. Table 1 illustrates this with respect to AD and how its typology category can change depending on the diagnostic criteria.
Table 1.
Relationship between Alzheimer disease conceptualizations, risk for dementia, and typology categories.
AD: Alzheimer disease; ADRDA: Alzheimer's Disease and Related Disorders Association; IWG: International Working Group; MCI: mild cognitive impairment; NA: not applicable; NIA-AA: National Institute on Aging–Alzheimer's Association; NINCDS: National Institute of Neurological and Communicative Disorders and Stroke.
aAssuming postmortem confirmation of AD-specific pathology is attained only in cases with dementia. Other than genetic testing for familial AD, the table also assumes that biomarkers are not available for diagnosis.
Typology category:
A. Asymptomatic person without dysfunction and not meeting diagnostic criteria for a disease.
B. Asymptomatic person without dysfunction but meeting diagnostic criteria for a disease.
C. Asymptomatic person with dysfunction not meeting diagnostic criteria for a disease.
D. Asymptomatic person with dysfunction meeting diagnostic criteria for a disease.
E. Symptomatic person without dysfunction and not meeting diagnostic criteria for a disease.
F. Symptomatic person without dysfunction meeting diagnostic criteria for a disease.
G. Symptomatic person with dysfunction not meeting diagnostic criteria for a disease.
H. Symptomatic person with dysfunction meeting diagnostic criteria for a disease.
Diagnostic criteria influence a multitude of factors; in the United States a diagnosis is required for insurance reimbursement; diagnosis guides treatment; diagnosis labels a person, for better or worse; and diagnostic criteria provide the basis for identification of cases of a disease, enabling research on specific diseases (exemplified by the inclusion/exclusion criteria of clinical trials, which often list specific diagnoses).
3. The typology
Take the following factors as the basis for a typology of clinical conditions, not all of which (according to the BST) are diseases.
-
1.
Symptomatic/asymptomatic
-
2.
Dysfunction/no dysfunction (dysfunction = statistical subnormality of internal physiological function)
-
3.
Meet/do not meet diagnostic criteria for a disease
Because each factor is dichotomous, eight (23) categories result:
-
A.
Asymptomatic person without dysfunction and not meeting diagnostic criteria for a disease: This is a healthy person.
-
B.
Asymptomatic person without dysfunction but meeting diagnostic criteria for a disease: This could be a risk-based condition (called a “risk-based disease” by Schwartz [2008] when there is no dysfunction), such as osteoporosis, hypertension, hypercholesterolemia, or type 2 diabetes.
-
C.
Asymptomatic person with dysfunction not meeting diagnostic criteria for a disease: This is a person with disease, not exhibiting symptoms, who remains undiagnosed. Examples include a person in the early stages or with a silent state of myasthenia gravis, type 2 diabetes, AD, Huntington disease, or any other disease wherein the pathophysiological processes begin years in advance of clinical presentation. For example, a person could have significant neurofibrillary tangles or Aβ plaques without having symptoms of AD.
-
D.
Asymptomatic person with dysfunction meeting diagnostic criteria for a disease: Compensated, controlled, or asymptomatic disease, whether as the result of treatment or the natural course of the disease. Examples include multiple sclerosis or Crohn disease in remission, or an infectious disease carrier.
-
E.
Symptomatic person without dysfunction and not meeting diagnostic criteria for a disease: Symptoms being part of normal variation, such as in a cough, bereavement, fatigue after work, or some cases of enuresis.
-
F.
Symptomatic person without dysfunction meeting diagnostic criteria for a disease: This is a person meeting diagnostic criteria that rely on the presence of symptoms, but who does not actually have a disease. Many of the disorders listed in the DSM-5 could fall under this category.
-
G.
Symptomatic person with dysfunction not meeting diagnostic criteria for a disease: This is a mild state of a disease, such as early stages of type 2 diabetes or the prodromal stage of Huntington disease. This could also occur where there are not good or accurate diagnostic criteria.2 This category is similar to ‘C’.
-
H.
Symptomatic person with dysfunction meeting diagnostic criteria for a disease: This is a typical diseased person.
Because A, B, E, and F do not involve dysfunction, these categories are not disease. As shown by categories C and D, dysfunction can be present even in asymptomatic disease. In cirrhosis, for example, a large amount of liver cells may be damaged, yet physical examination and liver function tests can still be normal because of the presence of healthy remaining liver cells. Other examples abound:
Diabetes, whether or not it is evident to its bearer, consists of an unusual deficiency in insulin secretion and therefore in sugar metabolism. Hepatic cirrhosis, nephritis, pancreatic cancer, and countless other pieces of local pathology can progress for a long time without depressing gross functions enough to be detected. They do, however, make standard tissue functions decline and fail in the affected part of the organ. (Boorse, 1977, p. 560)
Similarly, there are diseases (categories C, D, G, and H) for which asymptomaticity represents pathophysiological variability, in the sense that some cases of the disease result in symptoms, whereas others result in asymptomatic disease, but disease nonetheless (as assessed by signs, such as results of laboratory tests).
Including infectious-disease carriers in category D reflects the fact that there is dysfunction even though a carrier may by asymptomatic. On the BST and by the lights of the typology such carriers would be diseased. However, Wakefield argues that an asymptomatic infectious disease carrier is not diseased (2014, p. 664). He offers the case of Typhoid Mary, acknowledging that dysfunction was present, but denying that she was diseased because there was no symptomatic harm to her (although there was harm to others). Boorse recognizes that Wakefield's view of disorder as a harmful dysfunction is similar to his own view of therapeutic abnormality (1997, p. 49): “with dysfunction analyzing the “disease” part and harm analyzing the “clinical” part.” However, not all symptoms cause harm. Treatment, by contrast, involves normative notions, so it is interesting to note that multiple sclerosis, even if asymptomatic, may still be treated (in the hopes of slowing progression), whereas a carrier of herpes simplex virus type 1 typically is not treated if they do not have a cold-sore outbreak, despite what could involve a potential reduction in the transmissibility to others. A multitude of factors thus influences the decision to treat, which partly rests on the distinction between (potential) harm to self and (potential) harm to others. Similarly, there are other categories of clinical conditions, such as category F, where disease is not present yet normative considerations still play an important role. For example, drapetomania (Caplan et al., 2004) is not a disease because it involves no dysfunction, yet the decision to “treat” an absconding slave would definitely involve a normative consideration—in this case, quite an evil one.
An advantage of the BST is that it highlights the need for treatment for certain chronic, asymptomatic diseases well ahead of when they become clinically apparent. As Boorse stated (writing in 1987 [p. 370], but still relevant for today), “Current disease classifications, now grounded in a century of scientific physiology and pathology, reveal a converse danger in the clinical approach: it favors acute symptomatic disorders over more serious chronic ones.” By accounting for asymptomatic disease, the BST offers a conceptually robust basis for evaluating and guiding how diagnostic criteria are set for diseases. For example, it points to the presence of diseases for which some patients are diagnosed even if they do not have any dysfunction (category B, and possibly category F if the symptoms present are not reflective of any dysfunction). These include the risk-based conditions, discussed next.
4. Risk-based conditions
Some conditions straddle the line between disease and risk for disease (Doust et al., 2017; Rogers & Walker, 2017). Peter H. Schwartz (2008) identifies a number of diseases—hypercholesterolemia, type 2 diabetes, obesity, hypertension, osteoporosis—where a person could meet the diagnostic criteria for the disease yet not have an underlying dysfunction. Instead, they could be at risk for more serious manifestations of the disease. A risk-based condition could thus be defined as a clinical condition for which a person could meet diagnostic criteria for a “disease” yet for which in some cases no dysfunction is present (it is not a disease by the BST). The diagnostic criteria are better seen as biomarkers of risk for more severe disease or other diseases. What distinguishes risk-based conditions from non–risk-based conditions is the lack of dysfunction present in risk-based conditions for some patients meeting the diagnostic criteria. Typically, risk-based conditions are those for which overdiagnosis is a problem. This is because some cases of risk-based conditions should be “better understood as risk factors for developing disease in the future” (Schwartz, 2017, p. 497).
Although all the risk-based conditions Schwartz identifies are continuous-variable “diseases”,3 not all continuous-variable “diseases” are risk-based conditions. Hypothyroidism, for example, presents similar issues as with other continuous-variable “diseases”, but because the range is so narrow for which dysfunction is present, there may be fewer cases of overdiagnosis than in type 2 diabetes or hypertension. Moreover, mild elevation of thyroid stimulating hormone might not always reflect dysfunction but could be a sign of normal aging (Surks & Hollowell, 2007). Schwartz (2014a) equates pathology with dysfunction, which is different than suboptimal or below-average function. Type 2 diabetes, for example, becomes a disease when dysfunction is present but is still a disease for many HbA1c levels (i.e., the high ones), just not those that do not involve any underlying dysfunction. Risk-based “diseases” as Schwartz terms them is thus a misnomer on the typology (they should be called “risk-based conditions”) because it is only on current expanded-boundary diagnostic criteria that they are (wrongly according to Schwartz) considered diseases. Risk-based conditions show how a condition can straddle the boundary between one and another typology category (B and D). When diagnostic criteria lower the threshold by which a person is considered to have a disease, more people who are not diseased may be classified as being diseased, resulting in overdiagnosis and the medicalization of normal variation.
Élodie Giroux (2015, p. 188) charges Schwartz with not paying enough attention to the notion of functional efficiency with respect to risk-based conditions, and thinks he is unable to clarify whether a risk-based condition is normal or pathological. She thinks that medium or severe hypertension in itself is probably not a dysfunction. Schwartz (2008) considers these levels dysfunctional, in contrast to mild hypertension which he considers to not involve dysfunction. Giroux’s (2015, p. 188) critique is motivated by the divergence between blood pressure as a quantitative variable representing the level of functional efficiency and as a direct measurement of dysfunction of the circulatory system. Because she is correct that blood pressure is not a direct measurement of the dysfunction (i.e., functional efficiency) of the circulatory system (in most cases at least), she cannot find a basis for saying that any degree of hypertension represents dysfunction. Indeed, hypertension, as well as blood glucose levels, could be indications of disease, although they are also potentially pathogenic themselves (such as with glucotoxicity or when blood pressure gets really high, as pointed out by Boorse [2012]). Nonetheless, it is possible that there is dysfunction not only in medium or severe hypertension but even in mild hypertension. The dysfunction may just not rise to the level of clinically apparent dysfunction. There may still be dysfunction (such as at the level of the cell or molecule), that is undetectable with current clinical and diagnostic tools. But as Boorse (2012) notes, although there may be reduced functional efficiency in mild hypertension, if this is not age-excessive, then it does not count as disease on the BST. In summary, conflation of risk for disease or potential indicators of dysfunction, such as some levels of blood pressure, with dysfunction itself, can result in erroneous attribution of disease, a point to which diagnostic criteria should be sensitive if they are to accurately characterize a condition.
Risk-based conditions are only one of several types of clinical conditions possible based on the interplay among symptomaticity, dysfunction, and meeting of diagnostic criteria. Preclinical disease is another.
5. Preclinical disease: categories C and G
Another clinical condition for which risk for another disease or more severe disease plays a strong part involves mild, preclinical disease states for which diagnostic criteria for the disease are not met (in contrast with risk-based conditions where diagnostic criteria are met but there is no dysfunction). These other clinical conditions are categories C and G. Dysfunction is present, but not enough to cause symptoms. Thus, “preclinical” is a misnomer if it is meant to mean failure to meet diagnostic criteria and not simply the absence of symptoms, because by virtue of the presence of certain signs (such as biochemical signatures of the disease) some diagnostic criteria can be met (i.e., those based on signs), just not any symptom-based criteria.
Because preclinical disease creates something of a penumbral state, diagnostic criteria for some diseases explicitly incorporate symptom-based criteria, without which the person does not have the disease. However, doing so is not always advisable depending on the disease. In myasthenia gravis, for example, symptoms are helpful in reaching a diagnosis. In fact, a myasthenia gravis specialist, because of cost and other issues, may almost never encounter a patient tested for antibodies prior to entering the clinic with suspected myasthenia gravis (Prof. Dr. Joseph Bergmans, personal communication). Clinicians will typically diagnose (or are able to diagnose) a suspected case of myasthenia gravis even without antibody testing, solely on the basis of clinical presentation and/or electromyography results (Prof. Dr. Joseph Bergmans, personal communication). Yet to date no consensus group has promulgated diagnostic criteria requiring symptoms for myasthenia gravis. This makes sense for a disease like myasthenia gravis where a pathognomonic feature is present—antibody positivity—because no patients who are antibody positive will lack the disease.
Regarding AD, if AD were diagnosed solely on the basis of symptoms and not neuropathology, a person presenting with AD symptoms could not fall under category C. This is because category C involves asymptomatic people with dysfunction who do not meet diagnostic criteria for a disease. Were AD's diagnostic criteria to include laboratory evidence of AD neuropathology, however, then an asymptomatic person with AD could fall under category C if they were to not meet all the diagnostic criteria. This could happen if the person were to possess AD pathology not detectable by current technology (i.e., they still have dysfunction) or if they were to lack some AD symptoms that might still be required for an AD diagnosis (i.e., alongside the neuropathological evidence).
Importantly, this clinically unapparent dysfunction is not the same as risk for frank dysfunction. It could increase such risk, however, in the same way as any disease could increase the risk for another disease. This clinically unapparent dysfunction is still disease though—disease that current diagnostic tools just have not caught up with yet. For example, 100 years ago a person with the Huntington disease expansion still had Huntington disease even if unaware of their carrier status and not yet symptomatic. A similar situation exists for familial AD, because people with such genetic mutations are virtually guaranteed to develop dementia, the symptomatic hallmark of AD (Dubois et al., 2014, p. 615). Yet AD presents an even more intriguing test case for the typology than Huntington disease. This is because most cases of AD are of the sporadic type (Dubois et al., 2016, p. 298) and not all AD-biomarker–positive people develop cognitive impairment (Jack et al., 2018, p. 538). Nonetheless, recently proposed research diagnostic criteria allow for an AD diagnosis based entirely on biomarker findings (Dubois et al., 2016; Jack et al., 2018). Although these new diagnostic criteria are intended for research, some clinicians around the world do use biomarkers as an aid to diagnosis (Frisoni et al., 2017). This means some AD-diagnosed people could fall under category B or D, depending upon whether biological dysfunction is present. The implications of this are discussed next.
6. An illustrative example: Alzheimer disease and shifting typology categories
AD is a complex, multicausal condition clinically involving mild cognitive impairment (MCI) or dementia, and neuropathologically involving the presence of neurofibrillary tangles and senile plaques composed of Aβ (Vinters, 2015). AD offers an example of how the typology can help understand factors important for research, treatment, diagnosis, and nosology.
First, to know whether there is a theoretical disease present per the BST it is necessary to know the population distribution of the functional efficiency of a physiological part or process in a reference class (Boorse, 2014). Taking the elderly as a reference class, the pathophysiological hallmarks of AD, whether at the symptomatic or presymptomatic stage, would only count as theoretical disease if they are not commonly found in the appropriate reference class. For the purposes of this discussion I will assume this to be true, although given the high prevalence of AD pathology in individuals aged greater than 80 years (Jack et al., 2018, p. 552) this presumption is rebuttable.
Regarding diagnosis, since the 1984 publication of the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA; McKhann et al., 1984), AD was defined clinically on the basis of neuropsychological tests and a patient's symptoms. AD was therefore a syndrome (collection of symptoms). The presence of AD neuropathology was simply presumed (Jack et al., 2018), with only autopsy providing a definite confirmation. However, the existence of individuals with AD pathology but no AD symptoms, and AD symptoms but no AD pathology (Jack et al., 2018, p. 538), called into question this presumption. Although autopsy can confirm an AD diagnosis, this is rarely done now given the availability of in vivo biomarkers previously validated against postmortem AD pathology. Moreover, biomarker-based diagnostic criteria have recently been proposed for AD, enabling diagnosis independent of clinical symptoms, such as in the predementia phases. Brain biopsy is rarely performed to diagnose AD because it has low diagnostic yield—not only because it is too difficult and risky (not least of which from general-anesthesia sequelae) but because it is not sensitive and specific enough (Venneti et al., 2011; Warren et al., 2005).
In the last few years, with the advent of more sensitive biomarkers (specifically, those based on cerebrospinal fluid analysis and imaging, such as fluorodeoxyglucose positron emission tomography), a tension has sprung up between the 1984 clinical diagnostic criteria and newly promulgated research-based diagnostic criteria. For example, in 2011 the National Institute on Aging–Alzheimer's Association (NIA-AA) offered revised AD diagnostic criteria. These criteria viewed AD no longer as a syndrome but rather as a clinical–biomarker entity. Distinct diagnostic guidelines for AD's symptomatic stages—MCI and dementia (i.e., the full-blown clinical picture of AD)—were created, and these stages were still defined on the basis of clinical criteria. However, a new preclinical stage was defined that could be diagnosed mostly or entirely on the basis of biomarkers (though in contrast to MCI and dementia such diagnoses were to be used for research purposes only as biomarkers continued to develop [Jack et al., 2011]).
With the recognition that early treatment, even before clinical onset, could result in a greater chance to arrest the disease than with later treatment (Langbaum et al., 2013, p. 371; Graham, Bonito-Oliva, & Sakmar, 2017, p. 414; Jack et al., 2018, p. 548; Dubois et al., 2014, p. 620), there have been intense efforts to use biomarkers to identify people, even if currently asymptomatic, who would likely develop dementia or prodromal AD. This has resulted in a tension within AD diagnostic criteria (or, alternately put, a lack of consensus in the AD field as to what the diagnostic criteria should be), between symptom/neuropsychological-testing–based criteria and biomarker-based criteria. This tension is borne out in different recommendations for research diagnostic criteria promulgated by AD research groups, such as the research framework of the NIA-AA (Jack et al., 2011, 2018) and that of the International Working Group (IWG)/Dubois AD diagnostic criteria (Dubois et al., 2014, 2016). The latest NIA-AA framework defines AD as a biological entity, solely by its pathophysiology as evidenced by biomarkers, without consideration of symptoms (Jack et al., 2018). This allows for a biomarker-based diagnosis in asymptomatic people. By contrast, the IWG AD diagnostic criteria require both symptoms and biomarkers (Dubois et al., 2014). This could entail the existence of asymptomatic-at-risk-for-AD patients or presymptomatic patients, the latter carrying proven AD autosomal dominant mutations, which, because they have virtually complete penetrance, merit the “pre-” designation on the justified assumption that all such patients will develop dementia (Dubois et al., 2014, p. 621). A 2016 publication (Dubois et al., 2016) allowed for a diagnosis of preclinical AD in the absence of symptoms, aligning the criteria with the NIA-AA's view of AD as a biological, not syndromal, entity.
These developments in diagnostic criteria do more than change how AD is identified—they go to the heart of how AD is defined. Is AD a syndrome, a distinct type of neuropathology, or both? The distinction between a condition's diagnostic criteria and the definition of a condition is an important one, one the typology brings into sharp relief. This is by virtue of the symptomatology and dysfunction factors, because whereas a condition's definition may in some cases coincide with current diagnostic criteria, this is not always so. These cases of contested definitions (such as with AD) offer philosophically interesting and ethically important tests of whether biological dysfunction is more important than symptoms for defining a disease.
The 2018 NIA-AA research framework views dementia as a syndrome, not a disease, which is caused by AD (among other diseases): “The term “Alzheimer's disease” refers to an aggregate of neuropathologic changes and thus is defined in vivo by biomarkers and by postmortem examination, not by clinical symptoms” (Jack et al., 2018, p. 538). Yet this can be problematic because AD pathophysiological processes do not always lead to dementia. Indeed, in cognitively unimpaired people aged greater than 80 years, up to 60% have AD neuropathology (Jack et al., 2018, p. 552), and it is possible that these people do not develop dementia simply because they do not live long enough.
In the terms of the typology, positive cases of AD (i.e., those meeting the diagnostic criteria) have gone from being categories F, G, or H to being categories A, B, C, or D when asymptomatic and E, F, G, or H when symptomatic. Yet, as alluded to earlier, analyzed on the BST the asymptomatic or even prodromal stages may not be diseases at all, because the biological dysfunction may be too common, a point recognized by others (Alexopoulos & Kurz, 2015, p. 363; Shermer & Richard, 2018). Instead, they would be risk states.
Although there are some benefits to separating the pathological features from the clinical features (Jack et al., 2018), this has not been without its share of criticism, some authors going so far as to call the separation a messy divorce (McCleery, Flicker, Richard, & Quinn, 2019, p. 175). Indeed, reconceptualizing AD as solely a biological entity privileges a disease approach instead of an illness approach. It could even result in a worry-type illness state characterized by anxiety over the putative certainty of developing dementia, compounded by the negative societal consequences of being labeled as having AD. In addition to challenging common notions of dementia as understood by lay people, cultural differences in how dementia is perceived around the world (Alladi & Hachinski, 2018), as well as the unavailability of biomarkers in poorer regions, may make the reconceptualization of AD practical for only a minority of people. The ethically problematic nature and potential harm of asymptomatic or prodromal AD diagnoses include insurance, driving, and employment implications; additional costs for diagnostics and potentially ineffective treatments; needless worry and hypervigilance; and the stigma of being labeled with an AD diagnosis (Le Couteur, Doust, Creasey, & Brayne, 2013; Milne et al., 2018; Shermer & Richard, 2018; Swallow, 2017). Turning people who may or may not have a disease into patients with a chronic illness (Bedson, McCarney, & Croft, 2004) or patients-in-waiting (Timmermans & Buchbinder, 2010) similarly deserves careful deliberation, whatever the benefits the new research diagnostic criteria may hold. Reconceptualizing AD as various states of risk may help avoid some of these problems, but still can result in a “diagnosis” of risk, which can be harmful given that interventions to reduce risk can also cause harm (Accad & Fred, 2010).
By contrast, there could be benefits to conceiving AD solely as a biological entity. As mentioned, category C allows for a biomarker-based diagnosis of AD. If an AD diagnosis depended on clinical symptoms, then a large number of asymptomatic people who have the pathophysiological defects of AD might not be considered for treatment because they would not be diagnosed. This is particularly important given that the window for affecting the pathogenesis of AD via treatment may exist years before symptoms become apparent.
Applied to the clinic, there are risks with this approach to diagnosis and treatment, however. This is especially true when the line between physiological and pathological changes is difficult to distinguish. For example, in cardiomyopathy, technological advances have resulted in identification of asymptomatic, nonhypertrophic, genotype-positive people. Such individuals are at risk of being overdiagnosed because they are unlikely to become symptomatic or have deadly arrhythmias (Quarta et al., 2017). With respect to AD, diagnosis not dependent upon clinical symptoms is also subject to overdiagnosis if there are people with the pathophysiological defects who do not go on to develop AD symptoms. This could happen if the pathophysiological defects are normal signs of aging or if abnormal AD biomarkers are also present in old age, which in fact they are (Vinters, 2015).
Indeed, Alexopoulous and Kurz think there is practical validity in defining preclinical states of AD, such as by its effect on protective behaviors across the lifespan. However, they propose using the term “advanced brain aging” instead of “preclinical AD” on the grounds that they think a biomarker-based conceptualization of AD is conceptually invalid (Alexopoulos & Kurz, 2015). Although they are correct that AD biomarkers should be sufficiently validated and accurately distinguish between AD dementia and normal aging, AD neuropathology has been shown in a substantial number of brains of people aged less than 30 years (Braak & Del Tredici, 2011; Braak, Thal, Ghebremedhin, & Del Tredici, 2011; although, to be fair, not all such people will necessarily go on to develop AD symptoms in their old age). Moreover, the pathology associated with AD has been shown to not be due to aging-related mechanisms (Nelson et al., 2011). These findings highlight that the pathophysiological deficits of AD can begin well ahead of clinical symptoms.
While clinical dementia may sometimes be simply a product of normal aging, the dementia of AD is not. Several studies have shown that there are significant AD brain lesions in people at early stages of clinical cognitive symptoms (reviewed in Vinters, 2015). The reason recent trials of AD therapies may have failed is because only symptomatic patients were enrolled, thus excluding the chance to arrest the disease by addressing the pathophysiological defects in their incipient stages when therapy might have been more effective (Lyon, 2017; Langbaum et al., 2013, p. 371; Graham et al., 2017, p. 414; Jack et al., 2018, p. 548; Dubois et al., 2014, p. 620), a fact underscored by the oftentimes sudden onset of severe symptoms, the varying rate of disease progression among patients, and the sometimes decades-long prodromal phase, which can complicate measurement of drug efficacy (Graham et al., 2017, p. 425). Moreover, given AD's phenotypic, endotypic, and etiologic heterogeneity, characterizing the condition as a single disease is likely to lead to research and therapeutic dead-ends, such as with single-therapy solutions (Huang & Mucke, 2012).
How then should AD, or any other disease, be defined? Defined according to the BST on the basis of biological dysfunction, only biomarker-assessed neuropathology is needed for an AD diagnosis. But this does not mean that all diseases need to be recognized by clinical medicine (Tresker, 2020). Diagnostic criteria for AD that require symptoms in addition to biological dysfunction mitigates against the chance the person is merely a “carrier” and will never be affected in any significant way by the pathophysiology. However, each disease presents its own set of considerations, which militates against cookie-cutter solutions. Although whether a disease involves readily identifiable pathological signs might help, on its own this is insufficient to guide the setting of diagnostic criteria (i.e., deciding what criteria should be used by which a person can be diagnosed as having a specific disease). What is also needed is the use of normative considerations to dictate what criteria are used (Tresker, 2020).
7. Further implications for research and clinical practice: Risk
As shown with AD, understanding a condition's place in the typology may sensitize one to whether it may be possible to diagnose well before symptoms appear. For example, the beta-cell dysfunction and insulin resistance of type 2 diabetes have been hypothesized to exist years before glucose levels become elevated (Kahn, 2003). Type 2 diabetes and other conditions can also stress the line between disease and risk. For example, Vickers, Basch, and Kattan (2008) propose unambiguous lesions as distinguishing disease from risk. They give the example of a torn aorta. However, this should not be taken to mean that type 2 diabetes could not have unambiguous lesions. In the case of a torn aorta the lesion is at the level of the organ. With type 2 diabetes unambiguous lesions could be at the level of the cell or lower.
The idea of risk is complex when it comes to AD because it could refer to many things: the increased risk that APOE ε4 carriers, for example, have for developing AD (Dubois et al., 2016, p. 304) but which is by no means large enough to guarantee that all or most carriers will develop AD (whether defined via symptoms or solely by biomarkers). Other risk states, listed roughly in order of likelihood of resulting in dementia, include an asymptomatic person with some biomarker evidence of AD pathology, to such a person with more biomarker evidence, to a patient in the prodromal stage without biomarker evidence, to a prodromal patient with biomarker evidence, to a person with presymptomatic AD (i.e., familial AD), even if that person is not currently symptomatic (Table 1). The nature of the biomarker evidence results in different amounts of risk (Jack et al., 2016).
A key difference between the NIA-AA research framework and the IWG criteria is that whereas the former views asymptomatic but AD-biomarker–positive people as at risk for developing AD symptoms, the latter views these people as being at risk for developing AD (Jack et al., 2018, p. 551). This can conflict with lay intuitions about AD, a point recognized (but perhaps not adequately resolved) by the NIA-AA:
… we also recognize the deeply engrained historic use of the term “Alzheimer” to denote particular syndromes. Thus, we strongly recommend that a clinically ascertained syndrome consistent with what has historically been labeled “probable or possible AD” be referred to as Alzheimer's clinical syndrome, but not as AD or some modified form of AD (e.g., “possible or probable AD”). (Jack et al., 2018, p. 552)
The key to differentiating when conditions such as AD should be viewed as risk instead of disease may be the confidence with which diagnostic methods can unequivocally identify if and when the asymptomatic cases will turn into symptomatic cases and the prodromal cases will turn into manifest cases (e.g., dementia). High confidence might call for viewing as disease whereas low confidence might call for viewing as risk. However, this is easier said than done and the typology cannot directly help in this regard.
8. Conclusions
A direct consequence of the BST's conception of disease is its ability to distinguish cases where there is a disparity between the diagnostic criteria for a disease and the dysfunction putatively underlying the disease. Recognition of this can contribute to robust research programs that lead to revised diagnostic criteria for diseases. It can also affect nosology, leading to the splitting of one disease into many, or the unification of previously unrelated diseases (e.g., as with tuberculosis) when a common underlying etiology is identified. The result can be earlier diagnosis and more timely, effective interventions. In the case of AKI, for example, “[f]uture definitions [of AKI] are likely to incorporate novel functional and damage biomarkers to characterize AKI better” (Ostermann & Joannidis, 2016, p. 11).
As technology advances, the ability to probe into the pathophysiological defects of many diseases may improve, such that they can be treated, even if asymptomatic and representing just mild dysfunction. Such proactive intervention well ahead of when a person becomes symptomatic may also make it possible to modify the disease process and hopefully avert the development of the disease, or mitigate certain aspects of its presentation. This is now true for rheumatoid arthritis, for which disease-modifying drugs are available. For other diseases, such as atherosclerosis and type 2 diabetes, early lifestyle interventions (exercise, diet) have been shown to prevent their development or progression. For diseases in which there are neither cures nor effective treatments, such as Huntington disease, the value of knowing in advance whether one has the disease may be less than for diseases in which therapeutic interventions are available. Indeed, the rate of presymptomatic testing for Huntington disease is very low (Oster, Shoulson, & Dorsey, 2013). Nonetheless, in Huntington disease detectable pathophysiological changes are present one to two decades prior to clinical diagnosis (Paulsen et al., 2008). Research trials aimed at preventive therapeutics can thus still benefit from this lag between the beginning of pathophysiology and the appearance of symptoms. The existence of risk-based conditions—category B—which in some cases medicalize individual variation, could lead to less diagnosis of disease (if recognized as not involving dysfunction), whereas for category C diseases it could lead to more diagnosis (if diagnostic criteria are changed to allow for laboratory evidence of pathology). Understanding the nature of the categories could provide a conceptual toolkit for nosology and the setting of diagnostic criteria. This can lead to approaches that are directly relevant to patients. For example, patients falling within certain categories could be provided direct indicators of disease rather than treating risk factors for disease. Measuring and using carotid artery total plaque area as a guide to therapy, for instance, has been shown to improve therapy in cardiovascular disease prevention clinics (Spence & Hackam, 2010). Similarly, measurement of intima-media thickness affected both physician and patient behaviors, leading to better treatment behaviors (Korcarz et al., 2008). Making visible direct indicators of disease can in a way turn a sign into a symptom (i.e., the feeling engendered by witnessing the sign), with possible effects on an affected patient's motivation now that they can more palpably (or visually) experience a disease they may not yet suffer from.
As a classificatory system, the typology has several beneficial features: it is simple, containing only eight categories; exhausts the conditions clinicians encounter; has mutually exclusive categories; is flexible, able to accommodate new conditions and diseases; and is compatible with and can be integrated with other classification systems, such as the International Statistical Classification of Diseases and Related Health Problems (ICD). There are, however, some limitations. Although the typology can be conceptualized as a framework to help medical researchers, philosophers, bioethicists, and others think through considerations surrounding risk, disease, and the classification of medical conditions, the typology might only make explicit approaches that are already utilized, such as direct application of the notion of risk to classify a condition as a disease or not. The typology has not been empirically tested for its usefulness. Furthermore, the typology is silent on etiology, although it is fully compatible with classification systems that rely on etiology.
Ultimately, a key argument of this paper (and one for which a typology is not even needed), is that normative considerations—although absent on the conception of disease used here—do guide how disease is recognized in clinical practice. Here the typology may be useful by showing how normative considerations determine whether biological dysfunction or symptoms are more important for a condition's diagnostic criteria. Tensions between the relative importance of the typology's factors for a given condition can result in category reassignment, which can have enormous implications for how a condition is diagnosed, reimbursed by insurance, and viewed by patients. This is especially true when clinicians, patients, or researchers hold implicit biases against certain categories with respect to how conditions falling under those categories should be managed as a class. Further work can clarify the degree to which this is true.
In conclusion, by attending to the types of clinical conditions possible on the basis of three key features (i.e., symptomaticity, dysfunction, and the meeting of diagnostic criteria), clinical conditions as currently classified can be better categorized, highlighting the issues pertaining to certain typology categories. Hopefully this can reduce overdiagnosis or treatment where none is merited and at the same time increase sensitivity to risk for those individuals for whom it is important. This way such individuals’ conditions can be considered by medicine with just as much importance as disease in which dysfunction is readily apparent.
CRediT authorship contribution statement
Steven Tresker: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing - all drafts.
Declaration of competing interest
None.
Acknowledgments
I thank Christopher Boorse for helpful comments on this article. I thank two reviewers for this journal for their reviews, especially one reviewer for many helpful comments. The generous support of the Fonds Voor Wetenschappelijk Onderzoek-Vlaanderen (FWO) is acknowledged (1130819N).
I put “diseases” in scare quotes because while some continuous-variable conditions are diseases by the BST, some are not.
I thank an anonymous reviewer for this journal for this point.
References
- Accad M., Fred H.L. Risk-factor medicine: An industry out of control? Cardiology. 2010;117:64–67. doi: 10.1159/000319617. [DOI] [PubMed] [Google Scholar]
- Alexopoulos P., Kurz A. The new conceptualization of Alzheimer's disease under the microscope of influential definitions of disease. Psychopathology. 2015;48:359–367. doi: 10.1159/000441327. [DOI] [PubMed] [Google Scholar]
- Alladi S., Hachinski V. World dementia. Neurology. 2018;91:264–270. doi: 10.1212/WNL.0000000000005941. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. 2013. Diagnostic and statistical manual of mental disorders. Washington, DC. [Google Scholar]
- Bedson J., McCarney R., Croft P. Labelling chronic illness in primary care: A good or a bad thing? British Journal of General Practice. 2004;54:932–938. [PMC free article] [PubMed] [Google Scholar]
- Boorse C. Health as a theoretical concept. Philosophy of Science. 1977;44:542–573. [Google Scholar]
- Boorse C. Concepts of health. In: Van De Veer D., Regan T., editors. Health care ethics: An introduction. Temple University Press; Philadelphia: 1987. p. 359‒393. [Google Scholar]
- Boorse C. A rebuttal on health. In: Humber J.M., Almeder R.F., editors. What is disease? Humana Press; Totowa: 1997. pp. 1–134. [Google Scholar]
- Boorse C. 2012. Clinical normality. (Unpublished manuscript) [Google Scholar]
- Boorse C. A second rebuttal on health. Journal of Medicine and Philosophy. 2014;39:683‒724. doi: 10.1093/jmp/jhu035. [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K. Alzheimer's pathogenesis: Is there neuron-to-neuron propagation? Acta Neuropathologica. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- Braak H., Thal D.R., Ghebremedhin E., Del Tredici K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. Journal of Neuropathology and Experimental Neurology. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Caplan A.L., McCartney J.J., Sisti D.A., editors. Health, disease, and illness: Concepts in medicine. Georgetown University Press; Washington, D.C.: 2004. [Google Scholar]
- Doust J., Walker M.J., Rogers W.A. Current dilemmas in defining the boundaries of disease. Journal of Medicine and Philosophy. 2017;42:350–366. doi: 10.1093/jmp/jhx009. [DOI] [PubMed] [Google Scholar]
- Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow J. Advancing research diagnostic criteria for Alzheimer's disease: The IWG-2 criteria. The Lancet Neurology. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimer's and Dementia. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt H.T., Jr. 2nd ed. Oxford University Press; New York: 1996. The foundations of bioethics. [Google Scholar]
- Frisoni G.B., Boccardi M., Barkhof F., Blennow K., Cappa S., Chiotis K. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. The Lancet Neurology. 2017;16:661–676. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- Giroux É. Epidemiology and the bio-statistical theory of disease: A challenging perspective. Theoretical Medicine and Bioethics. 2015;36:175–195. doi: 10.1007/s11017-015-9327-7. [DOI] [PubMed] [Google Scholar]
- Graham W.V., Bonito-Oliva A., Sakmar T.P. Update on Alzheimer's disease therapy and prevention strategies. Annual Review of Medicine. 2017;68:413–430. doi: 10.1146/annurev-med-042915-103753. [DOI] [PubMed] [Google Scholar]
- Hausman D.M. Health, naturalism, and functional efficiency. Philosophy of Science. 2012;79:519–541. [Google Scholar]
- Hausman D.M. Health and functional efficiency. Journal of Medicine and Philosophy. 2014;39:634–647. doi: 10.1093/jmp/jhu036. [DOI] [PubMed] [Google Scholar]
- Hofmann B. Medicalization and overdiagnosis: Different but alike. Medicine, Healthcare & Philosophy. 2016;19:253–264. doi: 10.1007/s11019-016-9693-6. [DOI] [PubMed] [Google Scholar]
- Hofmann B. The overdiagnosis of what? On the relationship between the concepts of overdiagnosis, disease, and diagnosis. Medicine, Healthcare & Philosophy. 2017;20:453–464. doi: 10.1007/s11019-017-9776-z. [DOI] [PubMed] [Google Scholar]
- Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimer's and Dementia. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- Kline A.D. Health, disease and medicalization. International Journal of Applied Philosophy. 1986;3:85–88. [Google Scholar]
- Korcarz C.E., DeCara J.M., Hirsch A.T., Mohler E.R., Pogue B., Postley J. Ultrasound detection of increased carotid intima-media thickness and carotid plaque in an office practice setting: Does it affect physician behavior or patient motivation? Journal of the American Society of Echocardiography. 2008;21:1156–1162. doi: 10.1016/j.echo.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum J.B., Fleisher A.S., Chen K., Ayutyanont N., Lopera F., Quiroz Y.T. Ushering in the study and treatment of preclinical Alzheimer disease. Nature Reviews Neurology. 2013;9:371–381. doi: 10.1038/nrneurol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur D.G., Doust J., Creasey H., Brayne C. Political drive to screen for pre-dementia: Not evidence based and ignores the harms of diagnosis. BMJ. 2013;347:f5125. doi: 10.1136/bmj.f5125. [DOI] [PubMed] [Google Scholar]
- Lyon J. Alzheimer outlook far from bleak. Journal of the American Medical Association. 2017;317:896–898. doi: 10.1001/jama.2017.0276. [DOI] [PubMed] [Google Scholar]
- McCleery J., Flicker L., Richard E., Quinn T.J. When is Alzheimer's not dementia—Cochrane commentary on the National Institute on Ageing and Alzheimer's Association Research Framework for Alzheimer's Disease. Age and Ageing. 2019;48:174–177. doi: 10.1093/ageing/afy167. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Md Ralib A., Pickering J.W., Shaw G.M., Endre Z.H. The urine output definition of acute kidney injury is too liberal. Critical Care. 2013;17:R112. doi: 10.1186/cc12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R., Bunnik E., Diaz A., Richard E., Badger S., Gove D. Perspectives on communicating biomarker-based assessments of Alzheimer's disease to cognitively healthy individuals. Journal of Alzheimer's Disease. 2018;62:487–498. doi: 10.3233/JAD-170813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.T., Head E., Schmitt F.A., Davis P.R., Neltner J.H., Jicha G.A. Alzheimer's disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathologica. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbert C.M., Gala G.J. Supervenience and psychiatry: Are mental disorders brain disorders? Journal of Theoretical & Philosophical Psychology. 2015;35:203–219. [Google Scholar]
- Ostermann M. Diagnosis of acute kidney injury: Kidney Disease Improving Global Outcomes criteria and beyond. Current Opinion in Critical Care. 2014;20 doi: 10.1097/MCC.0000000000000157. 581‒7. [DOI] [PubMed] [Google Scholar]
- Ostermann M., Joannidis M. Acute kidney injury 2016: Diagnosis and diagnostic workup. Critical Care. 2016;20:299. doi: 10.1186/s13054-016-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster E., Shoulson I., Dorsey E.R. Optimal expectations and limited medical testing: Evidence from Huntington disease. The American Economic Review. 2013;103:804–830. doi: 10.1257/aer.106.6.1562. [DOI] [PubMed] [Google Scholar]
- Paulsen J.S., Langbehn D.R., Stout J.C., Aylward E., Ross C.A., Nance M. Detection of Huntington's disease decades before diagnosis: The Predict-HD study. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta G., Papadakis M., Donna P.D., Maurizi N., Iacovoni A., Gavazzi A. Grey zones in cardiomyopathies: Defining boundaries between genetic and iatrogenic disease. Nature Reviews Cardiology. 2017;14:102–112. doi: 10.1038/nrcardio.2016.175. [DOI] [PubMed] [Google Scholar]
- Reid S., Wessely S., Crayford T., Hotopf M. Medically unexplained symptoms in frequent attenders of secondary health care: Retrospective cohort study. BMJ. 2001;322:1–4. doi: 10.1136/bmj.322.7289.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilmann R., Leavitt B.R., Ross C.A. Diagnostic criteria for Huntington's disease based on natural history. Movement Disorders. 2014;29:1335–1341. doi: 10.1002/mds.26011. [DOI] [PubMed] [Google Scholar]
- Rogers W.A., Walker M.J. The line-drawing problem in disease definition. Journal of Medicine and Philosophy. 2017;42:405–423. doi: 10.1093/jmp/jhx010. [DOI] [PubMed] [Google Scholar]
- Schwartz P.H. Risk and disease. Perspectives in Biology and Medicine. 2008;51:320–334. doi: 10.1353/pbm.0.0027. [DOI] [PubMed] [Google Scholar]
- Schwartz P.H. Small tumors as risk factors not disease. Philosophy of Science. 2014;81:986–998. [Google Scholar]
- Schwartz P.H. Reframing the disease debate and defending the biostatistical theory. Journal of Medicine and Philosophy. 2014;39:572–589. doi: 10.1093/jmp/jhu039. [DOI] [PubMed] [Google Scholar]
- Schwartz P.H. Progress in defining disease: Improved approaches and increased impact. Journal of Medicine and Philosophy. 2017;42:485–502. doi: 10.1093/jmp/jhx012. [DOI] [PubMed] [Google Scholar]
- Shermer M.H.N., Richard E. On the reconceptualization of Alzheimer's disease. Bioethics. 2018;33:138–145. doi: 10.1111/bioe.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J.D., Hackam D.G. Treating arteries instead of risk factors: A paradigm change in management of atherosclerosis. Stroke. 2010;41:1193–1199. doi: 10.1161/STROKEAHA.110.577973. [DOI] [PubMed] [Google Scholar]
- Stegenga J. Effectiveness of medical interventions. Studies in History and Philosophy of Biological and Biomedical Sciences: Part C. 2015;54:34–44. doi: 10.1016/j.shpsc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Surks M.I., Hollowell J.G. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: Implications for the prevalence of subclinical hypothyroidism. Journal of Clinical Endocrinology & Metabolism. 2007;92:4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- Swallow J. Expectant futures and an early diagnosis of Alzheimer's disease: knowing and its consequences. Social Science & Medicine. 2017;184:57–64. doi: 10.1016/j.socscimed.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Timmermans S., Buchbinder M. Patients-in-waiting: Living between sickness and health in the genomics era. Journal of Health and Social Behavior. 2010;51:408–423. doi: 10.1177/0022146510386794. [DOI] [PubMed] [Google Scholar]
- Tresker S. Theoretical and clinical disease and the biostatistical theory. Studies in History and Philosophy of Biological and Biomedical Sciences: Part C. 2020 doi: 10.1016/j.shpsc.2019.101249. [DOI] [PubMed] [Google Scholar]
- Venneti S., Robinson J.L., Roy S., White M.T., Baccon J., Xie S.X. Simulated brain biopsy for diagnosing neurodegeneration using autopsy-confirmed cases. Acta Neuropathologica. 2011;122:737–745. doi: 10.1007/s00401-011-0880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers A.J., Basch E., Kattan M.W. Against diagnosis. Annals of Internal Medicine. 2008;149:200–203. doi: 10.7326/0003-4819-149-3-200808050-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinters H.V. Emerging concepts in Alzheimer's disease. Annual Review of Pathology. 2015;10:291–319. doi: 10.1146/annurev-pathol-020712-163927. [DOI] [PubMed] [Google Scholar]
- Wakefield J.C. The biostatistical theory versus the harmful dysfunction analysis, part 1: Is part-dysfunction a sufficient condition for medical disorder? Journal of Medicine and Philosophy. 2014;39:648–682. doi: 10.1093/jmp/jhu038. [DOI] [PubMed] [Google Scholar]
- Warren J.D., Schott J.M., Fox N.C., Thom M., Revesz T., Holton J.L. Brain biopsy in dementia. Brain. 2005;128:2016–2025. doi: 10.1093/brain/awh543. [DOI] [PubMed] [Google Scholar]