Fig. 4. Reductive amination of prochiral ketones.
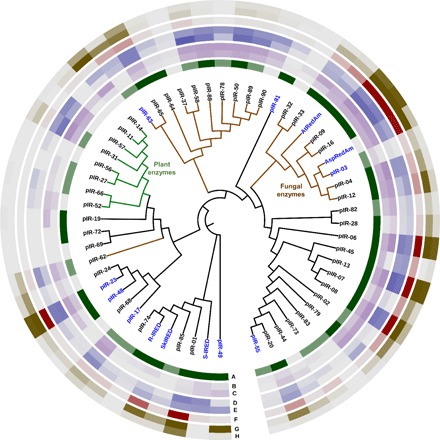
(A) Heterologous expression in E. coli; (B) conversion to 9 with 10 mM 1-indanone and 200 mM propargylamine at 30°C (up to 28%); (C) conversion to 9 with 10 mM 1-indanone and 200 mM propargylamine at 37°C (up to 37%); (D) conversion to 10 with 10 mM acetophenone and 200 mM cyclopropylamine at 30°C (up to 59%); (E) conversion to 10 with 10 mM acetophenone and 100 mM cyclopropylamine at 37°C (up to 50%); (F) conversion to 11 with 10 mM 4-hydroxyphenylacetone and 100 mM cyclopropylamine at 30°C (up to >99%); (G) conversion to 12 with 10 mM 4-methoxyphenylacetone and 100 mM cyclopropylamine at 37°C (up to >99%); and (H) conversion to 12 with 100 mM 4-methoxyphenylacetone and 200 mM cyclopropylamine at 37°C (up to 96%). Previously described IREDs are labeled in blue. Conversions were determined by GC-FID with comparison to chemically synthesized standards, with darker colors corresponding to higher conversions.
