Fig. 5. Rational mutagenesis for reductive amination with aniline.
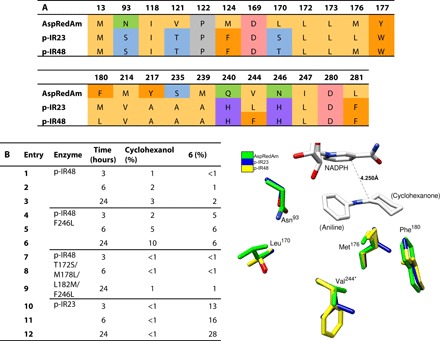
(A) Comparisons of theoretical extended active site residues for the reductive amination of cyclohexanone with aniline in AspRedAm to the analogous residues in the novel IREDs p-IR23 and p-IR48. (B) Conversions in the reductive amination of cyclohexanone with aniline with purified wild-type p-IR23, wild-type p-IR48, p-IR48 F246L, and p-IR48 T172S/M178L/L182M/F246L at different time points. Conversions were determined by GC-FID with comparison to a chemically synthesized standard. The adjacent image shows the positions of nonconserved residues with 6 docked in the active site, based on homology modeling. Residue 244* is attached to the second protein chain in the dimer.
