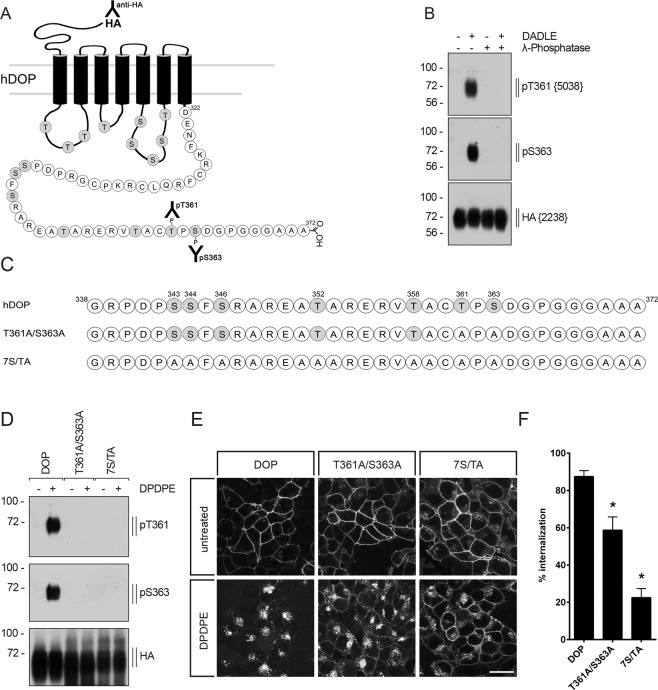
Figure 1.
Characterization of phosphosite-specific DOP receptor antibodies using λ-phosphatase and receptor mutants. (A) Schematic representation of the human DOP receptor (hDOP). Potential intracellular phosphorylation sites are depicted in gray. T361 was targeted for the generation of the phosphosite-specific anti-pT361 antibody and anti-pS363 antibody was acquired commercially. (B) Characterization of phosphosite-specific antibodies directed against T361 and S363 using λ-phosphatase. Stably HA-tagged hDOP-receptor expressing HEK293 cells were either not treated (−) or treated (+) with 10 µM DADLE for 10 min. Lysates were then either not incubated (−) or incubated (+) with λ-phosphatase and immunoblotted with the phosphosite-specific antibodies anti-pT361 {5038} or anti-pS363. Blots were stripped and reprobed with the anti-HA antibody {2238} as a loading control. Blots are representative, n = 3. Molecular mass markers (kDA) are indicated, left. (C) Sequence of the carboxyl-terminal tail of hDOP receptor showing all potential phosphorylation sites. Serine (S) and threonine (T) residues indicated were exchanged to alanine. (D) HEK293 cells stably expressing HA-hDOP receptor, T361A/S363A or 7 S/TA were not treated (−) or treated (+) with 1 µM DPDPE for 10 min and lysates were immunoblotted with the antibodies to pT361 or pS363. Blots were stripped and reprobed with the anti-HA antibody. Blots are representative, n = 5. (E) Cells described in (D) were preincubated with antibody to HA-tag and subsequently exposed to 10 µM DPDPE or vehicle for 30 min at 37 °C. Cells were fixed, permeabilized, immunofluorescently stained and examined using confocal microscopy. Images are representative from one of three independent experiments. Scale bar, 20 µm. (F) Receptor internalization was quantified by ELISA. Cells described in (D) were preincubated with antibody to HA-tag and stimulated with 10 µM DPDPE or vehicle at 37 °C for 30 min. Cells were fixed and labeled with a peroxidase-conjugated secondary antibody. Receptor internalization was measured by enzyme-linked immunosorbent assay and quantified as the percentage of internalized receptors in DPDPE-treated cells. Data are mean ± SEM of five independent experiments performed in quadruplicate. Results were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test (*p < 0.05).