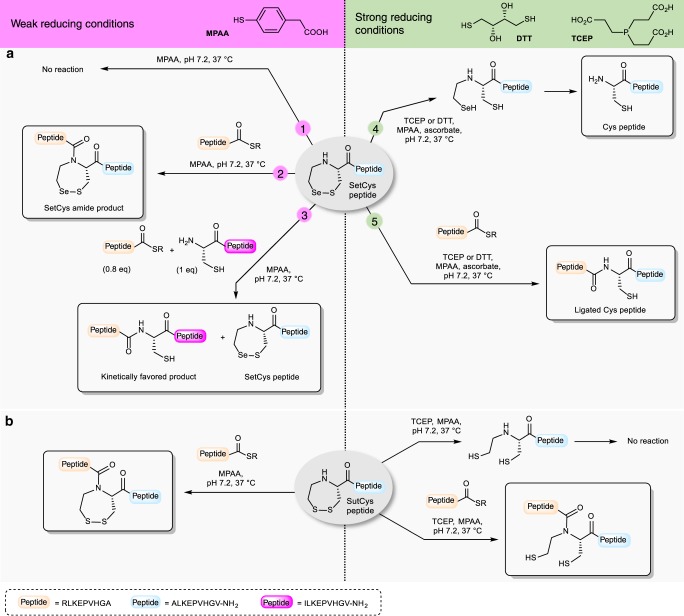
Fig. 2. Reactivity of N-(2-chalcogenoethyl)cysteine (SetCys and SutCys) peptides as a function of the reducing strength of the mixture.
a The reactivity of N-(2-selenoethyl)cysteine (SetCys) peptides is controlled by the reducing strength of the mixture. The numbers on the arrows indicate different experimental conditions. Conditions 1–3 (−SR = 3-mercaptopropionic acid, MPA): weakly reducing conditions, typically in the presence of an excess of 4-mercaptophenylacetic acid (MPAA) at neutral pH. Conditions 4 and 5 (−SR = MPA or MPAA): strong reducing conditions, typically in the presence of dithiothreitol (DTT) or tris(2-carboxyethyl)phosphine (TCEP) at neutral pH. b Ligation of an N-(2-sulfanylethyl)cysteine (SutCys) peptide with a peptide alkyl thioester under weak or strong reducing conditions.