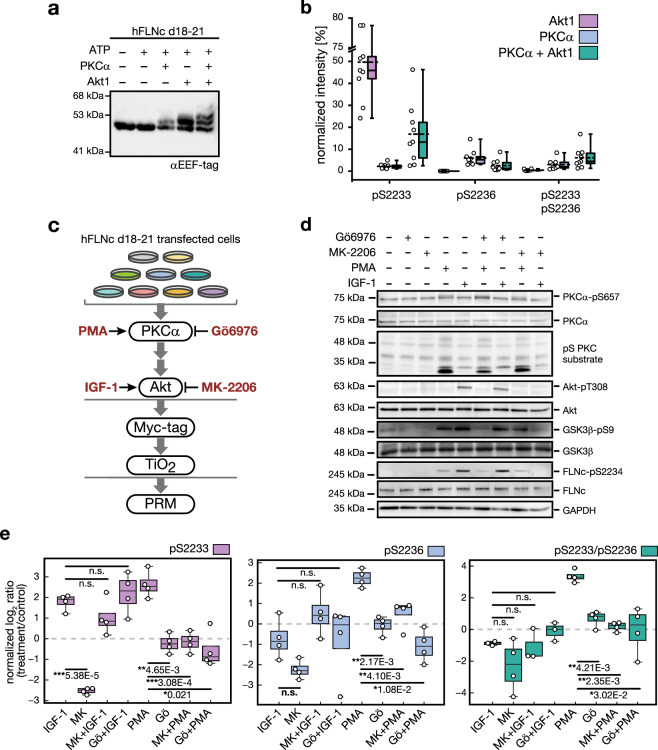
Fig. 4. Phosphorylations within the extended motif located in the insert of hFLNc domain 20 are mediated by Akt and PKCα.
a In vitro kinase assay coupled to phosphorylation-dependent mobility shift analysis. Reactions were performed using recombinant hFLNc d18–21 in the presence (+) or absence (−) of ATP and the two basophilic kinases Akt and PKCα. Immunoblot analysis was performed with an antibody directed against the EEF-tag that was fused to the carboxy-terminus of hFLNc d18–21. A specific phosphorylation-dependent mobility shift was detected following incubation with PKCα. Akt-mediated phosphorylation led to band shift of approximately 50% of the protein. Incubation with both kinases resulted in two shifted bands, indicating dual-site phosphorylation of the protein. d, domain. b In vitro kinase assay coupled to quantitative MS analysis for site determination. Reactions were performed as described in (a). MS data for each kinase were quantified using Skyline. Intensities of phosphopeptides distinctive for a specific phosphorylation site in hFLNc d18–21 were added up per experiment and represented as normalized mean ± SEM (n = 3 independent experiments, each with 3 technical replicates). c Cell-based kinase assay. PKC activator PMA, PKCα inhibitor Gö6976 and Akt activator IGF-1 and Akt inhibitor MK-2206 were used to activate or block signaling pathways in C2 cells. For targeted MS analysis, phosphopeptides were enriched by Myc-tag and TiO2-based enrichment. d Immunoblot analysis for monitoring the activity levels of PKCα and Akt in C2 cells following pharmacologic interventions as indicated in (c). Specific antibodies were used to detect total protein amounts and phosphoisoforms. GSK3β-pS9 is a direct substrate of Akt and pS PKC substrate is an antibody directed against PKC substrates. GAPDH was used as loading control. e Targeted MS data showing changes in phosphorylation of hFLNc at S2233 and S2236. For parallel reaction monitoring, the phosphopeptides LGpSFGSITR, LGSFGpSITR, LGpSFGpSITR, ERLGpSFGSITR and ERLGpSFGpSITR were selected. MS data were quantified using Skyline and normalized to an internal phosphopeptide standard and a two-tailed paired student’s t-test was performed. Shown are the normalized mean log2 ratios (treatment/control) ± SEM, n = 4 independent experiments.