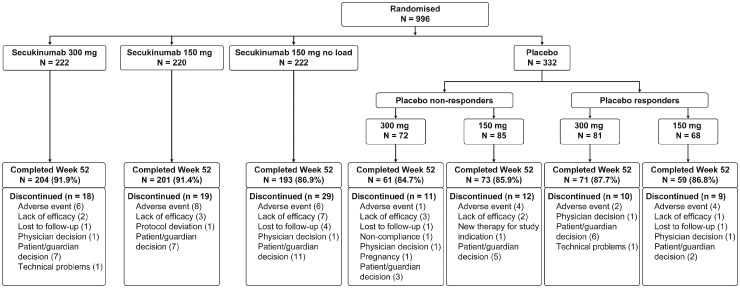
Fig. 1.
Patient disposition through week 52
Patients received s.c. secukinumab 300 or 150 mg, or placebo at baseline, weeks 1, 2 and 3, and every 4 weeks starting at week 4. Patients in the 150 mg no load arm were administered placebo at weeks 1, 2 and 3. At week 16, non-responders (<20% reduction in TJC and/or SJC) in the placebo group were switched to s.c. secukinumab 300 or 150 mg in a double-blind manner, and similarly, responders were switched at week 24. N: number of randomized patients; SJC: swollen joint count; TJC: tender joint count.