Figure 5. Coordinate regulation of developmental progression and cell fate transitions in C. elegans larvae.
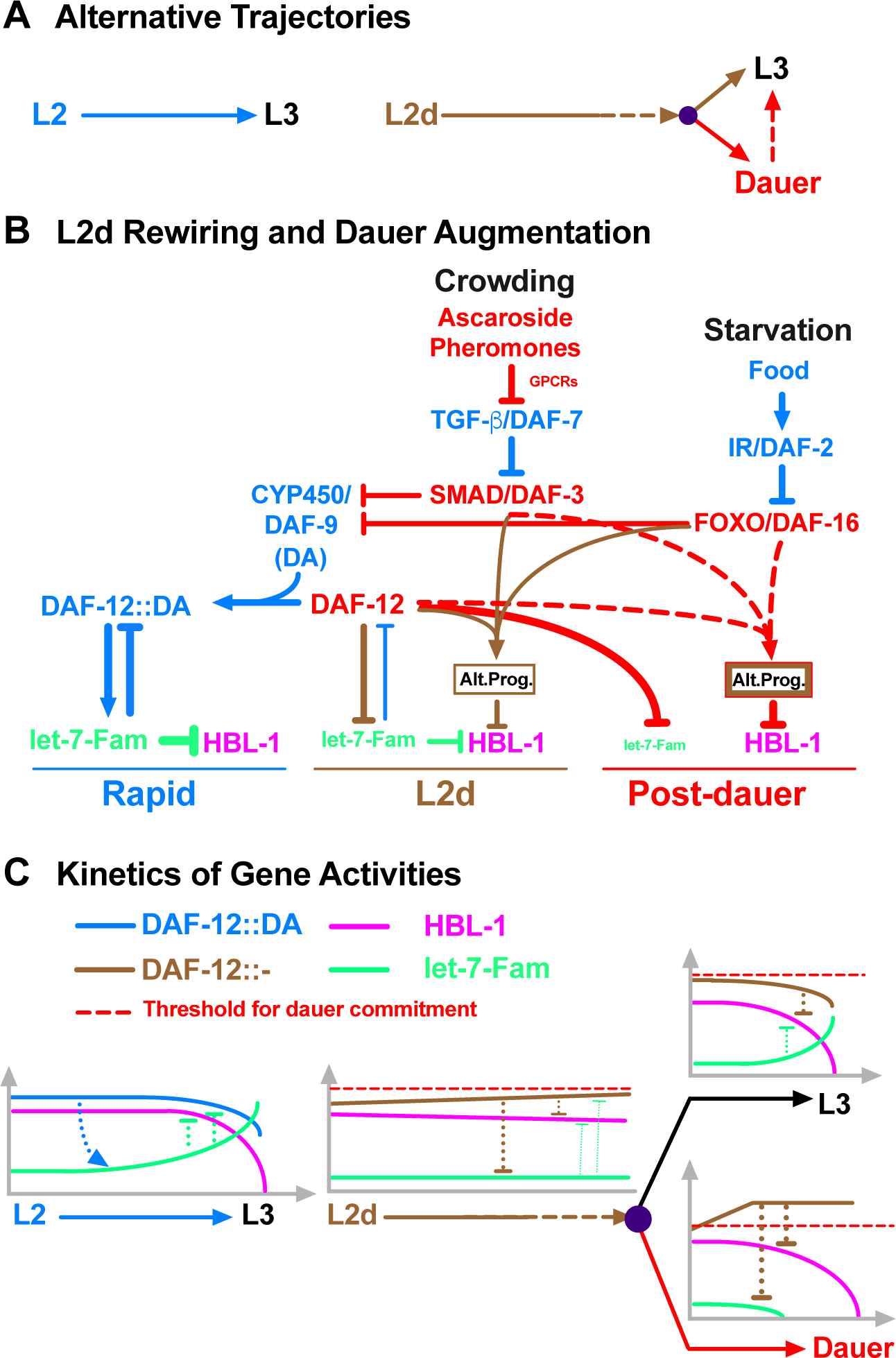
(A) Alternative trajectories. The L2-to-L3 transition is rapid and deterministic (once committed to the L2 stage, the larvae do not have the dauer option), whereas the L2d-to-L3 transition is slower and bipotential. In both cases, HBL-1 is present throughout the L2/L2d stage but it is downregulated by the beginning of the L3 stage (Figure S4). (B) Pheromone and endocrine signals engage DAF-12 to initiate and regulate the rewiring of the HBL-1 down-regulation. In response to crowding and starvation, TGF-β and insulin signaling pathways, respectively, modulate the ligand-dependent DAF-12 activity to repress the transcription of let-7 family microRNAs, and, at the same time, cooperate with DAF-12 in a ligand independent manner to activate the alternative HBL-1 downregulation program (Alt. Prog.). The alternative program of L2d and dauer-interrupted trajectories are similar, but the alternative program of dauer-interrupted trajectory is stronger either due to an enhancement of the alternative program of L2d (depicted as thicker brown border line) and/or due to employment of additional factors (depicted as red border line) after the L2d larvae commit to dauer formation. (C) DAF-12 ensures properly delayed but robust HBL-1 down-regulation during L2d-to-L3 transition by coordinating the repression of let-7 family microRNAs with the activation of the alternative HBL-1 downregulation program. During rapid, L2 development, DAF-12 activates the transcription of let-7 family microRNAs, which in turn, negatively regulate DAF-12, eliminating the dauer option. During slow, bipotential, L2d development, DAF-12 represses let-7 family microRNAs, which otherwise prevent the accumulation of DAF-12. If the unliganded DAF-12 reaches the threshold, larvae commit to dauer formation, if not, larvae commit to continuous development. While mediating this decision, which necessitates the repression of let-7 family microRNAs (for maintaining the dauer option) and delaying the downregulation of HBL-1 (for postponing L3 cell fates), DAF-12 cooperates with DAF-3 or DAF-16 to activate the alternative HBL-1 downregulation program (Alt. Prog) to ensure robust HBL-1 downregulation during the L2d-to-L3 transition. See also Figure S4.
