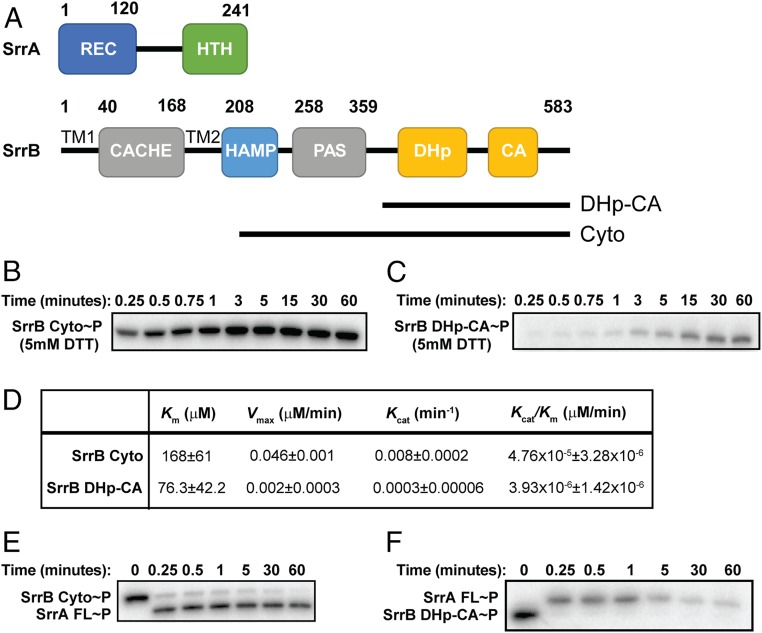
Fig. 1.
Domain architecture and regulation of SrrB activity by the PAS domain. (A) Domain architecture of SrrA and SrrB proteins. SrrB is a membrane-bound histidine kinase with extracellular Cache domain, transmembrane HAMP domain, intracellular PAS domain, and DHp-CA catalytic domain. SrrA is predicted to be an OmpR-like response regulator with receiver domain and winged helix-turn-helix DNA binding domain. (B) Autophosphorylation of SrrB HAMP-PAS-DHp-CA and (C) DHp-CA. (D) Kinetics of autophosphorylation of SrrB HAMP-PAS-DHp-CA and DHp-CA regions. Km, Vmax, and Kcat values were calculated by fitting the kinetics data to the Michaelis–Menten equation in GraphPad software. The errors represent the error of fitting the model to the data. Phosphotransfer of (E) SrrB HAMP-PAS-DHp-CA and (F) DHp-CA to full-length SrrA. Experiments were performed in triplicate, and representative gels are shown.