Abstract
Background
Duration of viral shedding is a determinant of infectivity and transmissibility, but few data exist about oseltamivir's ability to alter viral shedding.
Methods
From January 2012 through October 2017, a randomized, double-blinded multicenter clinical trial was conducted in adults aged 18–64 years at 42 sites in Thailand, the United States, and Argentina. Participants with influenza A or B and without risk factors for complications of influenza were screened for the study. Eligible participants were randomized to receive oseltamivir 75 mg or placebo twice daily for 5 days. The primary endpoint was the percentage of participants with virus detectable by polymerase chain reaction in nasopharyngeal swab at day 3.
Results
Of 716 adults screened for the study, 558 were randomized, and 501 were confirmed to have influenza. Forty-six participants in the pilot study were excluded, and 449 of the 455 participants in the population for the primary analysis had day 3 viral shedding results. Ninety-nine (45.0%) of 220 participants in the oseltamivir arm had virus detected at day 3 compared with 131 (57.2%) of 229 participants in the placebo arm (absolute difference of −12.2% [−21.4%, −3.0%], P =; .010). The median time to alleviation of symptoms was 79.0 hours for the oseltamivir arm and 84.0 hours for the placebo arm (P =; .34) in those with confirmed influenza infection.
Conclusions
Oseltamivir decreased viral shedding in this low-risk population. However, in the population enrolled in this study, it did not significantly decrease the time to resolution of clinical symptoms.
Clinical Trials Registration
Keywords: influenza-like illness, respiratory virus, viral shedding
The duration of viral shedding is thought to be a determinant of transmissibility [1], but few studies have specifically examined oseltamivir's ability to alter viral shedding and infectivity. Two pivotal adult studies that evaluated oseltamivir's effect on symptom resolution evaluated viral shedding. In one of these studies, oseltamivir treatment resulted in decreased viral shedding in a combined nose and throat swab. The viral shedding area under the curve (AUC) was 130 median tissue culture infectious dose (TCID50) × h/mL, 78 TCID50 × h/mL (P =; .03), and 94 TCID50 × h/mL (P =; .003) for the placebo, oseltamivir 75 mg twice daily, and oseltamivir 150 mg twice daily arms, respectively [2]. In the other adult licensing study, the difference in viral shedding was not statistically significant between any of the arms [3]. While the clinical efficacy of oseltamivir has previously been established, the present study was specifically designed to evaluate the virologic efficacy of oseltamivir.
METHODS
Trial Design and Study Population
The study was a randomized, double-blind study conducted in Thailand, the United States, and Argentina. Males and nonpregnant females aged 18–64 years with 1 or more respiratory symptoms (cough, sore throat, or nasal symptoms) starting no more than 48 hours before screening who did not have any underlying medical condition thought to increase the risk of complications from influenza and had an influenza A or B diagnosed locally by rapid antigen or polymerase chain reaction (PCR) were eligible for the study. The medical conditions associated with increased risk of complications from influenza that were exclusionary were age ≥65 years, presence of 1 or more chronic medical conditions (detailed in the Supplementary Materials), or body mass index ≥40 kg/m2.
Eligible participants were randomly assigned to treatment by an online computer-generated randomization system in a 1:1 ratio to receive either oseltamivir 75 mg or matching placebo given orally twice a day for 5 days. All participants, site staff, and the study team were masked to treatment allocation and remained blinded until after final database lock. The study protocol was approved by an institutional review board/ethics committee for each study site as well as by all local and national governing bodies as applicable. All study participants provided written informed consent. Additional methods are provided in the Supplementary Materials.
The first 50 participants randomized were part of a planned pilot study with frequent study visits to help determine the virologic endpoint to be used in the primary efficacy analysis based on virologic and practical considerations (actual N =; 46). These participants had nasopharyngeal (NP) and oropharyngeal (OP) swabs collected on days 1, 2, 3, 5, 7, 10, and 14, and all samples were tested using both PCR and TCID50. After the pilot study, the primary endpoint was chosen to be the percentage of participants shedding virus by PCR in NP swab on day 3.
Participant Recruitment and Duration of Follow-up
Participants were assessed on study day 0 (predose) and days 3, 7, and 28. NP swabs were collected from the participants by the study team on days 0, 3, and 7, and blood samples were collected on days 0, 3, 7, and 28. Participants received symptom diary cards that were to be completed twice daily from day 0 to day 7, once daily for days 8–14, and again on day 28 with the study team.
Study Outcomes
The primary endpoint was the percentage of participants shedding virus by PCR in NP swab on day 3. Secondary clinical endpoints included time to alleviation of influenza clinical symptoms (all symptoms grade 1 [mild] or absent) when assessing cough, nasal obstruction, sore throat, fatigue, headache, myalgia, feverishness, rhinorrhea, nausea, vomiting, and diarrhea (the first 7 symptoms were those assessed in previous licensure studies). Other secondary endpoints were the proportion of participants who developed sinusitis, otitis media, bronchitis/bronchiolitis, pneumonia, or other complications of influenza requiring antibiotics; answers to 2 global assessment questions; safety (grade 4 adverse events and serious adverse events), and 28-day mortality. The Data and Safety Monitoring Board reviewed the safety data from the study.
Virologic Analysis
Influenza type and subtype were determined using the Centers for Disease Control and Prevention protocol of real-time reverse transcriptase PCR (RT-PCR) for influenza A and B and performed at the Naval Health Research Center in San Diego, California. Influenza viral load and 2 housekeeping genes (human β-2-microglobulin [B2M] and glyceraldehyde-3-phosphate dehydrogenase were determined by quantitative PCR (qPCR) using the TaqMan method. See the Supplementary Materials for full virology methods.
Statistical Analyses
The sample size of 560 participants, which included 50 participants from the pilot study, was initially chosen assuming the primary endpoint selected after the pilot study was AUC viral shedding (log10 TCID50 × h/mL). After the pilot study, although the primary endpoint was changed, the same sample size was unchanged based on the fact that it would give approximately 90% power to detect an absolute difference of 15% in the proportion of participants with undetectable virus shedding at day 3 (eg, a reduction of 15% from 57.5% in the oseltamivir arm to 42.5% in the placebo arm) using a 2-sided type I error rate of 5% and allowing for 10% of participants to have unavailable viral shedding results at day 3.
The protocol-specified efficacy analyses are presented for all randomized participants with influenza infection confirmed by the central laboratory on a baseline NP sample who had received at least 1 dose of study medication (similar to prior studies [2, 3]). As the primary endpoint was chosen based on data from the pilot study, the protocol-specified primary endpoint analysis was further restricted to exclude all participants in the pilot study. The pilot study used to pick the primary endpoint did not evaluate any nonvirology endpoints. Therefore, the modified intention-to-treat (mITT) population, which consists of those randomized participants who took at least 1 dose of the study medication, is used for the study population characteristics, safety analysis, and all secondary clinical endpoints.
RESULTS
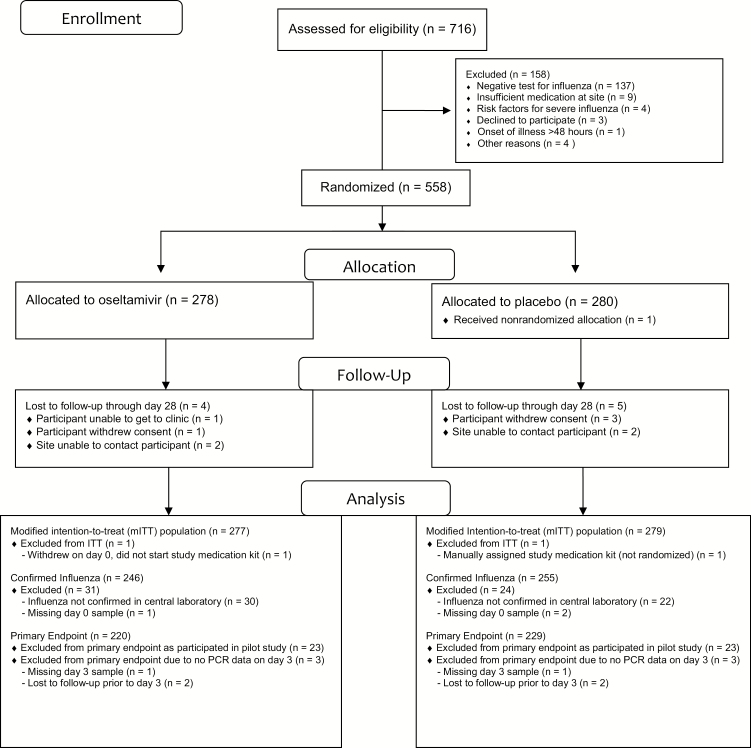
Between January 2012 and October 2017, 716 participants were assessed for eligibility for randomization in this study (Figure 1). Four participants were excluded: 3 were inadvertent enrollments (1 meant to be enrolled in another study [IRC003] [4], 1 was a participant enrolled under 2 identifying numbers, 1 was a practice enrollment [ie, dummy participant]), and 1 was a repeat enrollment, which was not allowed. Of the remaining 712 participants, 154 were excluded during screening; 137 (89%) did not have influenza demonstrated on a site screening test. Additional reasons for screening failure are noted in Figure 1. Two additional participants are excluded from the ITT population: 1 participant withdrew consent on day 0 prior to receiving study medication and the other inadvertently received a study medication kit from the site prior to randomization.
Figure 1.
Trial flow diagram. Abbreviations: ITT, intention-to-treat; PCR, polymerase chain reaction.
A total of 556 participants from 34 sites were included in the ITT population: 366 (66%) at 4 sites in Thailand, 178 (32%) at 27 sites in the United States, and 12 (2%) at 3 sites in Argentina. US sites did not enroll participants from March 2014 until April 2016 due to a sponsor decision to have these sites prioritize enrollment into a different influenza treatment trial (IRC003) [4]. No sites enrolled from April 2016 until February 2017 due to delays in manufacturing additional study medication kits. The study was stopped after reaching its planned enrollment in October 2017.
Characteristics of participants in the ITT population are described in Table 1. The median age of participants was 36 years, with a range of 18–64 years. Participants presented with a median of 28 hours of illness, were randomized a median of <1 hour after screening, and started study medication a median of 1 hour after randomization. All participants were positive with a local diagnostic test for influenza, of which 546 (98%) were using rapid antigen assays. By local testing, 387 (70%) had influenza A, 164 (29%) had influenza B, and 5 (1%) reported more than 1 subtype. By central laboratory qualitative PCR testing, 251 (45%) participants had influenza A/H3N2, 93 (17%) influenza A/H1N1, 156 (28%) influenza B, 1 participant had coinfection (qualitative PCR positive for H3N2 and B), and 52 (9%) were influenza-negative. The remaining 3 had no baseline sample available for testing. Therefore, 501 participants had confirmed influenza infection by central laboratory testing.
Table 1.
Baseline Characteristics of the Modified Intention-to-treat Population
| Characteristic | Total | Oseltamivir | Placebo |
|---|---|---|---|
| (N =; 556) | (n =; 277) | (n =; 279) | |
| Age, y | |||
| Median (quartiles) | 36 (27, 47) | 37 (27, 49) | 35 (27, 46) |
| Min, Max | 18, 64 | 18, 64 | 18, 63 |
| Sex | |||
| Female | 347 (62%) | 183 (66%) | 164 (59%) |
| Race | |||
| Asian | 385 (69%) | 193 (70%) | 192 (69%) |
| White | 150 (27%) | 74 (27%) | 76 (27%) |
| Black or African American | 18 (3%) | 7 (3%) | 11 (4%) |
| Race not available | 3 (1%) | 3 (1%) | 0 |
| Ethnicity | |||
| Hispanic or Latino | 24 (4%) | 11 (4%) | 13 (5%) |
| Country | |||
| Thailand | 366 (66%) | 182 (66%) | 184 (66%) |
| United States | 178 (32%) | 91 (33%) | 87 (31%) |
| Argentina | 12 (2%) | 4 (1%) | 8 (3%) |
| Influenza type (site testing) | |||
| A | 387 (70%) | 187 (68%) | 200 (72%) |
| B | 164 (29%) | 88 (32%) | 76 (27%) |
| Coinfection with more than 1 virus | 5 (1%) | 2 (1%) | 3 (1%) |
| Influenza type (central laboratory) | |||
| Influenza A/H3N2 | 251 (45%) | 122 (44%) | 129 (47%) |
| Influenza A/H1N1 | 93 (17%) | 39 (14%) | 54 (19%) |
| Influenza B | 156 (28%) | 85 (31%) | 71 (26%) |
| Negative | 52 (9%) | 30 (11%) | 22 (8%) |
| Coinfectiona | 1 (<0.5%) | 0 (0%) | 1 (<0.5%) |
| No. missing | 3 | 1 | 2 |
| Hours from onset of influenza-like illness to screening | |||
| Median (quartiles) | 28 (21, 39) | 29 (22, 40) | 27 (20, 37) |
| Min, Max | 0, 49 | 1, 49 | 0, 49 |
| Hours from screening to randomization | |||
| Median (quartiles) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Min, Max | 0, 23 | 0, 23 | 0, 10 |
| Hours from randomization to treatment initiation | |||
| Median (quartiles) | 1 (0, 5) | 1 (0, 5) | 1 (0, 5) |
| Min, Max | 0, 21 | 0, 20 | 0, 21 |
| Smoking (at any point)? | |||
| Yes | 68 (12%) | 35 (13%) | 33 (12%) |
| Influenza vaccination in the season of enrollment? | |||
| Yes | 57 (10%) | 34 (12%) | 23 (8%) |
aCoinfection: quantitative polymerase chain reaction (qPCR) results are for flu A/H3 as flu B was not detected by qPCR.
At baseline, participants were moderately symptomatic (median 13 points across 11 symptoms each scored 0–3 [absent–severe]; Table 2). Participants had some physical limitations as assessed on the SF-36 physical domain (median 65) and almost universally were not feeling as well or functioning as well as before they developed the influenza illness (531 [97%] and 461 [84%], respectively). Very few participants had any of the solicited complications from influenza (1% or less for each) or were taking antibiotics for nontargeted complications at baseline (2%; Table 2).
Table 2.
Baseline Symptoms and Virology of Participants With Influenza Confirmed in Central Laboratory Testing
| Symptoms and Virology | Total | Oseltamivir | Placebo |
|---|---|---|---|
| (N =; 501) | (n =; 246) | (n =; 255) | |
| Overall symptom score (11 symptoms, graded 0–3) | |||
| Median (quartiles) | 13 (10, 17) | 13 (10, 17) | 13 (10, 17) |
| Min, Max | 3, 27 | 3, 27 | 3, 27 |
| Average functional status (physical domain of the SF-36) | |||
| Prior to illness–median (Q1:Q3) | 100 (95, 100) | 100 (90, 100) | 100 (95, 100) |
| Day 0–median (Q1:Q3) | 65 (40, 75) | 65 (40, 75) | 65 (35, 75) |
| Global assessment | |||
| Participant feels as good today as before illness (no) | 531 (97%) | 260 (95%) | 271 (98%) |
| Participant functions as well today as before illness (no) | 461 (84%) | 224 (82%) | 237 (86%) |
| Complications at baseline | |||
| Sinusitis | 7 (1%) | 4 (1%) | 3 (1%) |
| Otitis media | 1 (<0.5%) | 0 (0%) | 1 (<0.5%) |
| Bronchitis/Bronchiolitis | 6 (1%) | 4 (1%) | 2 (1%) |
| Pneumonia | 1 (<0.5%) | 0 (0%) | 1 (<0.5%) |
| Using antibiotic for other reasons | 11 (2%) | 6 (2%) | 5 (2%) |
| Virology | |||
| Mean (standard deviation) log10 copies/mL | 6.6 (1.3) | 6.6 (1.3) | 6.6 (1.3) |
| Median (quartiles) log10 copies/mL | 6.9 (5.8, 7.6) | 6.9 (5.8, 7.6) | 6.9 (5.9, 7.6) |
| Detection on quantitative assay | |||
| ≥LLOQ | 482 (96%) | 237 (96%) | 245 (96%) |
| ≥LOD, <LLOQ | 11 (2%) | 6 (2%) | 5 (2%) |
| <LOD | 8 (2%) | 3 (1%) | 5 (2%) |
| Presence of glyceraldehyde-3-phosphate dehydrogenase | |||
| ≥LOD/LLOQ, =ULOQ | 493 (99%) | 243 (99%) | 250 (98%) |
| <LOD/LLOQ | 7 (1%) | 3 (1%) | 4 (2%) |
| Presence of B2M | |||
| ≥LOD/LLOQ, =ULOQ | 499 (100%) | 246 (100%) | 253 (99%) |
| <LOD/LLOQ | 2 (0%) | 0 (0%) | 2 (1%) |
Abbreviations: B2M, beta-2 microglobulin; LOD, lower limit of detection; LLOQ, lower limit of quantification; ULOQ, upper limit of quantification.
Of the 501 participants with confirmed influenza infection in central laboratory testing, 8 (2%) did not have virus detected on the qPCR assay and 11 (2%) had virus detected below the assay lower limit of quantification (LLOQ). Quantification of the housekeeping gene B2M in all the samples with an undetectable virus or viral level less than the LLOQ suggested that inability to quantify virus level was not due to poor sample collection technique. The median (quartiles) viral load at baseline was 6.9 (5.8, 7.6) log10 copies/mL (Table 3) and was similar in the 2 treatment arms. In the ITT population, 543 of the 556 participants (98%) reported taking all study doses on all 5 days; the proportion was similar in the 2 arms (270 [97%] in the oseltamivir arm vs 273 [98%] in the placebo arm). Losses to follow-up prior to 28 days were very low: 4 participants (1.4%) in the oseltamivir arm vs 5 (1.8%) in the placebo arm.
Table 3.
Influenza Virus Over Time in the Primary Efficacy Population
| Virologic Measurement | Total | Oseltamivir | Placebo | Extension of Wilcoxon Test |
|---|---|---|---|---|
| Day 0, n | 501 | 246 | 255 | … |
| Median (quartiles) log10 copies/mL | 6.90 (5.80, 7.60) | 6.85 (5.80, 7.60) | 6.90 (5.90, 7.60) | … |
| Day 3, n | 495 | 243 | 252 | 0.07 |
| Median (quartiles) log10 copies/mL | 3.90 (3.20, 4.70) | 3.40 (3.20, 4.50) | 3.90 (3.20, 4.90) | … |
| ≥LLOQ | 194 (39%) | 85 (35%) | 109 (43%) | … |
| ≥LOD, <LLOQ | 60 (12%) | 24 (10%) | 36 (14%) | … |
| <LOD | 241 (49%) | 134 (55%) | 107 (42%) | … |
| No. missing | 6 | 3 | 3 | … |
| Day 7, n | 491 | 241 | 250 | 0.46 |
| Median (quartiles) log10 copies/mL | 3.20 (3.20, 3.40) | 3.20 (3.20, 3.40) | 3.20 (3.20, 3.40) | … |
| ≥LLOQ | 37 (8%) | 16 (7%) | 21 (8%) | … |
| ≥LOD, <LLOQ | 16 (3%) | 11 (5%) | 5 (2%) | … |
| <LOD | 438 (89%) | 214 (89%) | 224 (90%) | … |
| No. missing | 10 | 5 | 5 | … |
Abbreviations: LOD, lower limit of detection; LLOQ, lower limit of quantification.
Of the 501 participants with confirmed influenza infection, 46 were in the pilot study and so excluded from the analysis of the primary endpoint. An additional 6 participants did not have a day 3 virologic endpoint sample available for testing (3 in each arm). Thus, 449 participants contributed results for the analysis of the primary endpoint. There were 99 (45.0%) of the 220 participants in the oseltamivir arm who had virus detected at day 3 compared with 131 (57.2%) of the 229 participants in the placebo arm (−12.2% difference; 95% confidence interval [CI], −21.4%, −3.0%; P =; .0097). This difference was larger in those enrolled within 24 hours of symptom onset (28/65 [43.1%] vs 61/97 [62.9%]) compared with those enrolled within 24–36 hours (38/77 [49.4%] vs 36/64 [56.3%] or 36–48 hours (33/78 [42.3%] vs 34/68 [50%]). The results of the primary endpoint were similar when those in the pilot study were included: 109 (44.9%) of 243 in the oseltamivir arm compared with 145 (57.5%) of 252 in the placebo arm (difference, −12.7%; 95% CI, −21.4%, −3.9%; P =; .0048).
For influenza type, there was a significant treatment-by-influenza-type interaction (interaction, P =; .012). Among participants with influenza type A infection, a smaller number of participants had detectable viral load in the oseltamivir arm (61 of 158 [38.6%] participants with results) vs placebo arm (108 of 182 (59.3%); difference, −20.7%). However, among participants with influenza B infection, there was little difference in the proportion with detectable viral shedding between the 2 arms: 48 of 85 (56.5%) in the oseltamivir arm and 37 of 70 (52.9%) in the placebo arm (difference, +3.6%). For the other 2 prespecified subgroup analyses, there were no significant interactions of treatment with sex (male vs female) or country/race (US/white vs US/nonwhite vs Thailand vs Argentina).
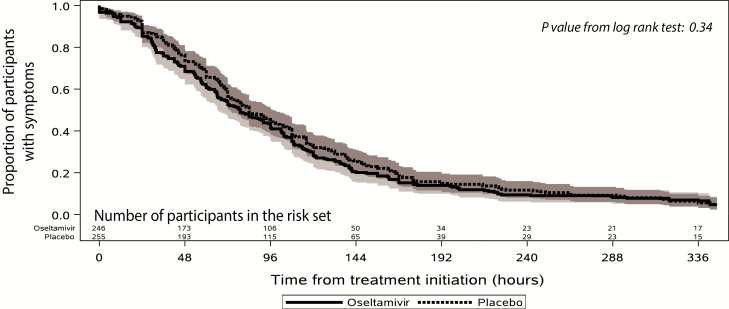
The median time to alleviation of symptoms was 79.0 hours (66.4–92.0) for the oseltamivir arm and 84.0 hours (73.3–97.3) for the placebo arm (P =; .34; Figure 2). The ITT analysis had similar results (82.0 hours compared with 84.1 hours, P =; .30). When calculated by half-day intervals matching the planned diary card assessment (recognizing the time of the diary card does not reflect the time of symptom resolution, but rather the time picked by the participant for completion of the diary card), the results were unchanged (3.5 days vs 4.0 days, P =; .35 in the primary efficacy population). Subgroup analyses by influenza type, sex, and country/race did not reveal any significant interaction with treatment. Analysis by duration of illness prior to study drug administration did not reveal significant differences in efficacy with regard to the duration of illness prior to study drug enrollment (interaction P value =; .40).
Figure 2.
Proportion of participants with clinical symptoms (in those with confirmed influenza infection).
The diary card included 2 global assessment questions. The median duration until the participant was feeling as well as before the influenza illness was 134.7 vs 133 hours among participants with confirmed influenza (P =; .27) (Supplementary Figure 1A). There was no significant difference between arms in median time to return to the preillness level of function (78.8 vs 103.5, P =; .36) (Supplementary Figure 1B). There was also no significant difference in the SF-36 physical function score between the 2 randomized arms at day 3, 7, 14, or 28 (Supplementary Figure 1C).
All samples that had influenza virus >LLOQ on day 7 were sequenced. Prior literature was reviewed to identify nucleotide substitutions associated with resistance [5–7]. No samples demonstrated a change in sequence at loci (compared with day 0 sample) known to be associated with resistance to oseltamivir.
DISCUSSION
We sought to evaluate the virologic efficacy of oseltamivir compared with placebo in a population at low risk of complications of influenza. In the primary analysis, those treated with oseltamivir were less likely to have viral RNA detectable in the nose on day 3 compared with those treated with placebo (absolute reduction of 12.2%).
Treanor et al evaluated viral shedding in 211 of 374 participants with influenza and was not able to demonstrate a difference at any time point [3]. Nicholson et al previously demonstrated a difference in the AUC of viral titers (78.2 vs 130.8 log10 TCID50 × h/mL, P =; .03) as measured by viral culture of nose and throat swabs at days 2, 4, 6, and 8 in 350 of 475 participants with influenza [2]. Our pilot study suggested that this intense sampling would compromise enrollment. Additionally, in the pilot portion of this study, 72% of day 3 samples tested by TCID50 were negative, necessitating the use of the proportion with detectable viral shedding by PCR on day 3 rather than TCID50 as the endpoint. In a similar study that involved a high-risk population [4], this percentage of day 3 samples testing negative by TCID50 was even higher at 97%. Our reduced study visit schema on days 0, 3, and 7 and the day 7 sample being undetectable in most (90%) participants necessitated use of the proportion with detectable viral shedding by PCR on day 3 rather than AUC for this study.
We were not able to demonstrate the same clinical benefit in this low-risk population among multiple clinical parameters as prior licensing studies [2, 3]. Cochrane performed a meta-analysis of 20 treatment trials that demonstrated considerable variation in the reduction of clinical symptoms after oseltamivir treatment, ranging from 8.4 to 25.1 hours [8]. Our results are in line with this, though we were still surprised by the minimal clinical efficacy. It is important to highlight that the population enrolled in this study and those populations enrolled in prior oseltamivir treatment studies used for licensure are not similar. Most notably, even though both enrolled influenza A and B, influenza B made up 28% of the current study and only 1%–3% of licensing studies. The efficacy of oseltamivir against influenza B has been questioned [9, 10], and our data indeed confirm minimal or no clinical or virologic efficacy against influenza B in a low-risk population.
The 2 large adult licensing studies used a cutoff for symptoms prior to enrollment of 36 hours and demonstrated a benefit in median time to symptoms resolution of 31.8–43 hours, whereas a demonstrated clinical benefit up to 48 hours after onset of symptoms was shown in other studies [11]. Oseltamivir was licensed for use within 48 hours of onset of symptoms, which is why this interval was used in this study. Early initiation of oseltamivir after onset of symptoms increases its therapeutic effects, though even those who initiated therapy at 36 hours after onset of illness were noted to have a 25% reduction in duration of illness [11]. In our study, the difference in duration of illness prior to enrollment did not appear to alter the clinical efficacy, though the study was not powered for these subgroup analyses. Our study had a similar requirement for symptoms as the adult licensing studies, though ours did not require fever for enrollment. The presence of fever may select for a population with more severe disease and more likely to benefit from oseltamivir. Additionally, our population may have been less symptomatic (median 13 points across 11 symptoms, compared with 14–15 across 7 symptoms in prior pivotal studies [2, 3]).
Our study also enrolled from a broader geographic area than prior licensing studies. It is possible that the symptoms assessed, previously tested in US and European populations, do not reflect the primary symptoms in other populations. Last, given practical considerations, participants were asked to record data on a diary card twice daily in this study. In a disease such as influenza that typically resolves within 3–4 days, using twice-daily assessments may be insensitive to detect rapid improvement.
The results from our study should not be used to raise a question about the utility of oseltamivir in most populations. This was not the primary objective of this study, and this study enrolled a very specific low-risk population. We believe that there are sufficient data from multiple prior studies, including many nonmanufacturer-sponsored observational studies, to support the conclusion that neuraminidase inhibitors including oseltamivir have clinical benefit and that current recommendations for their use in treating those with influenza that requires hospitalization, those who are very sick, and those who are at high risk of severe influenza complications are justified. The minimal virologic benefit and the nonsignificant clinical differences demonstrated in this study, however, should raise caution in automatically extending those recommendations to include treatment of a low-risk population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The following sites/investigators randomized participants in the IRC004 study: United States: Advanced RX Clinical Research Group, Westminster, CA. Dinh V. Dinh, DO; Bandera Family Health Care, San Antonio, TX. Ramon Reyes, MD; Clinical Research; Advantage, Inc., Council Bluffs, IA. Jennifer Kay, MD; Clinical Research Solutions, Franklin, TN. Alex Slandzicki, MD; Clinical Research Solutions, Jackson, TN. Melanie Hoppers, MD; Clinical Research Solutions, Middleburg Heights, OH. Lawrence Gervasi, MD; Clinical Research Solutions, Nashville, TN. Heather Rowe, MD; Clinical Research Solutions, Smyrna, TN. Sadia Dar, MD; Columbus Regional Research Institute, Columbus, GA. Jeffrey Jenkins, MD; DMI Research, Inc., Pinellas Park, FL. Bridget Bellingar, DO; Frontier Clinical Research, Smithfield, PA. Marcy Goisse, MD; Great Lakes Medical Research, Westfield, NY. Timothy Kitchen, MD; Health Concepts, Rapid City, SD. Richard L. Beasley, MD; National Institutes of Health—Clinical Center, Bethesda, MD. Richard Davey, MD; Paragon Rx Clinical, Inc., Garden Grove, CA. Hoang Nguyen, MD; Prairie Fields Family Medicine, Freemont, NE. Thomas A. Wolf, MD; Skyline Medical Center, Elkhorn, NE. William Fitzgibbons, MD; Texas Tech University Health Sciences Center at Amarillo, Amarillo, TX. Todd Bell, MD; University of Colorado, Aurora, CO. Michelle Barron, MD; University of Florida, Gainesville, FL. Marie-Carmelle Elie, MD; University of Iowa, Iowa City, IA. Patricia Winokur, MD; University of Pennsylvania, Philadelphia, PA. Pablo Tebas, MD; University of Southern California, Los Angeles, CA. Fred R. Sattler, MD; University of Texas Health Sciences Center at Houston, Houston, TX. Karen Vigil, MD; University of Virginia, Charlottesville, VA. Birgit Winther, MD; Village Health Partners, Plano, TX. Madhavi Ampajwala, MD; Westlake Medical Research, Thousand Oaks, CA. Krista Preston, DO. Thailand: Bamrasnaradura Infectious Diseases Institute, Nonthaburi, Thailand. Weerawat Manosuthi, MD; Khon Kaen University, Srinagarind Hospital, Khon Kaen, Thailand. Ploenchan Chetchotisakd, MD; King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Kiat Ruxrungtham, MD; Siriraj Hospital, Bangkok, Thailand. Winai Ratanasuwan, MD. Argentina: Hospital General de Agudos J. M. Ramos Mejía, Ciudad de Buenos Aires, Argentina. Marcelo Losso, MD; Hospital Municipal “Prof. Dr. Bernardo A. Houssay,” Pcia. de Buenos Aires, Argentina. Gustavo Lopardo, MD; Hospital Rawson, Córdoba, Argentina. Daniel David, MD In addition to the investigators and site staff, the following contributed significantly to the conduct of the IRC004 trial: National Institute of Allergy and Infectious Diseases: Julia Metcalf, Susan Vogel, Kelly Cahill, John Tierney, and Jerry Pierson Leidos Biomedical: Beth Baseler, Shelley Simpson, Mi Ha Kim, Olukayode Koleoso, Carla Chorley, Theresa Engel, Angel Gonzalez-Rodriguez, Aaron Vittini, Shawn Brown, Sharon Beck, Lisa Giebeig, Katelyn Patterson, Christina Burks, Devon Moore, and Ilmiya Yarullina. Social & Scientific Systems Inc: Nicholas Langlois, Sean McCarthy, Stephanie Warner, Catherine Morales, Jillian Van Zee, Casey Miller, Teresia Wanjiku, Yvette Delph, Dorothy O'Neil, and Nikki Gettinger. Harvard T.H. Chan School of Public Health: Justin Ritz and Evgenia Aga. Frontier Science Foundation: Kenneth Wood, Mark Byroads, Adam Manzella, Sean Yaeger, and Anthony Holguin. Naval Health Research Center, San Diego, CA: Patrick Blair, Dennis Faix, Melinda Balansay-Ames, Scott Vo, Roger Pan, Hayden Thammavong, and James Pethers. The Human Immunodeficiency Virus Netherlands Australia Thailand Research, Collaboration (HIV-NAT). Patumwan, Bangkok, Thailand. Kiat Ruxrungtham, MD and Anchalee Anchalee Avihingsanon, MD. Coordinación en Investigación Clínica Académica en Latinoamérica (CICAL), Buenos Aires, Argentina: Cecilia Abela, Alejandra Moricz and Marina Delfino. The authors also acknowledge the advice and assistance of the NIAID intramural Data Safety Monitoring Board in guiding the progress of the study: Data and Safety Monitoring Board (DSMB) Core Members: William Blackwelder, PhD (Chair), Carol Tacket, MD, Lawrence Moulton, PhD, David Parenti, MD, Mary Young, MD, Wilbur Chen, MD, James Baraniuk, MD (resigned June 2015), DSMB ad hoc Members: Pedro Politi, MD, Juan Carlos Tinoco, MD, David Smith, MBBS, Sasisopin Kiertiburanakul, MD, and Khuanchai Supparatpinyo, MD.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the US Department of the Navy, US Department of the Army, US Department of the Air Force, US Department of Veterans Affairs, US Department of Defense, or the US government. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. Employees of the sponsor of the study were involved with study design, analysis, and the writing of the report.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH). This project has been funded in part with federal funds from the National Cancer Institute, NIH, under contracts HHSN261200800001E and HHSN261201500003I.
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ryoo SM, Kim WY, Sohn CH, et al. . Factors promoting the prolonged shedding of the pandemic (H1N1) 2009 influenza virus in patients treated with oseltamivir for 5 days. Influenza Other Respir Viruses 2013; 7: 833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nicholson KG, Aoki FY, Osterhaus AD, et al. . Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet 2000; 355: 1845–50. [DOI] [PubMed] [Google Scholar]
- 3. Treanor JJ, Hayden FG, Vrooman PS, et al. . Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283: 1016–24. [DOI] [PubMed] [Google Scholar]
- 4. Beigel JH, Bao Y, Beeler J, et al. ; IRC003 Study Team Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: a multicentre, double-blind, randomised phase 2 trial. Lancet Infect Dis 2017; 17: 1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deyde VM, Sheu TG, Trujillo AA, et al. . Detection of molecular markers of drug resistance in 2009 pandemic influenza A (H1N1) viruses by pyrosequencing. Antimicrob Agents Chemother 2010; 54: 1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McKimm-Breschkin JL. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses 2013; 7(Suppl 1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheu TG, Deyde VM, Garten RJ, Klimov AI, Gubareva LV.. Detection of antiviral resistance and genetic lineage markers in influenza B virus neuraminidase using pyrosequencing. Antiviral Res 2010; 85: 354–60. [DOI] [PubMed] [Google Scholar]
- 8. Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ.. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ 2014; 348: g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sugaya N, Mitamura K, Yamazaki M, et al. . Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 2007; 44: 197–202. [DOI] [PubMed] [Google Scholar]
- 10. Burnham AJ, Baranovich T, Govorkova EA.. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral Res 2013; 100: 520–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aoki FY, Macleod MD, Paggiaro P, et al. ; IMPACT Study Group Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 2003; 51: 123–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.