Abstract
Exploration of animal models lead to discoveries that can reveal candidate biomarkers for translation to human populations. Herein, a model of hepatocarcinogenesis and protection was used in which rats treated with aflatoxin (AFB1) daily for 28 days (200 μg/kg BW) developed tumors compared with rats completely protected from tumors by concurrent administration of the chemoprotective agent, 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im). Differential expression of miRNAs in tumors (AFB1) and non-tumor (AFB1+ CDDO-Im) bearing livers and their levels in sera over the life-course of the animals was determined. miRNA transcriptome analysis identified 17 miRNAs significantly upregulated at greater than five-fold in the tumors. The ten most dysregulated miRNAs judged by fold-change and biological significance were selected for further study, including liver specific miR-122–5p. Validation of sequencing results by real-time PCR confirmed the upregulation of the majority of these miRNAs in tumors, including miR-182, as well as miR-224–5p as the most dysregulated of these miRNAs (over 400-fold). Longitudinal analysis of levels of miR-182 in sera demonstrated significant and persistent increases (5.13 fold; 95% CI: 4.59–5.70). The increase in miR-182 was detected months before any clinical symptoms were present in the animals. By the terminal time point of the study, in addition to elevated levels of serum miR-182, serum miR-122–5p was also found to be increased (>1.5 fold) in animals that developed hepatocarcinomas. Thus, using data from an unbiased discovery approach of the tissue findings, serum miR-182 was found to track across the complex, multistage process of hepatocarcinogenesis opening an opportunity for translation to human populations.
Keywords: miRNA, aflatoxin B1, CDDO-Im, RNA sequencing, hepatocarcinogenesis
1. Introduction
Hepatocellular carcinoma (HCC) continues to be a major health problem worldwide, claiming nearly 830,000 lives in 20161. Most HCC is conceptually preventable since the major risk factors have been characterized; however, mortality rates still remain high. This is partially due to the typical diagnosis of patients during the advanced stages of disease when symptoms are more apparent, but treatment is not often effective2. The 5-year overall survival rate for patients detected in early stages is over 50%, but that rate universally drops to less than 16% when diagnosis occurs at later stages3–5. It is crucial to have effective early surveillance to identify those who could benefit the most from therapeutic interventions. Current strategies include imaging and measuring serum α-fetoprotein (AFP) and des-gamma carboxyprothrombin (DCP) levels. Imaging by ultrasound or computed tomography (CT) is a primary method for tumor detection but these instruments may not be readily accessible in economically poor countries where the rates of HCC are among the highest in the world6,7. Finally, the serum biomarkers currently used have poor sensitivity and specificity during early stage disease further complicating prevention strategies5.
MicroRNAs (miRNAs, miRs) are short non-coding RNAs that can modulate the expression of target messenger RNAs (mRNAs). They have been demonstrated to be dysregulated in experimental and human HCC and have emerged as promising disease biomarkers because of their stability and sequence homology between species, especially in mammals8–14. MicroRNAs can be detected in the circulation15,16. Studies have identified a variety of miRNAs that differentiate HCC patients from healthy controls17–25. Over expression of tissue miRs-34a, 200a, 221 and serum miR-224 have been associated with poor prognosis in HCC patients11,17,26–28. Limited experimental information has been revealed about miRNAs in aflatoxin-induced HCC but several investigations to date have focused on an acute exposure period and genotoxic effects of aflatoxin on miRNAs29,30.
Our previous study identified a panel of miRNAs that were dysregulated in hepatic tissue and sera at the end of a 4-week carcinogenic AFB1 dosing regimen both with and without chemoprotection31. The miRNAs rno-miR-122–5p, 34a-5p, and 181c-3p were found to be increased in the livers and sera of animals dosed with AFB1. Animals co-exposed to the chemopreventive agent 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im) were protected from the genotoxic insult of aflatoxin and displayed near-basal hepatic expression of candidate miRNAs. CDDO-Im is an analog of the drug bardoxolone-methyl currently in clinical trials, and is a potent activator of Nrf2 signaling that leads to enhanced aflatoxin detoxication32,33. For the current study, we sought to determine dysregulated miRNAs that longitudinally emerge in sera during the lifetime of the animals and in the sera of the rats with HCC at termination. Initial discovery experiments used RNA sequencing and real-time PCR in tumor tissue induced by aflatoxin and non-tumor hepatic tissue from both untreated and AFB1 plus CDDO-Im co-exposed animals. The miRNA profile was dramatically altered in tumors when compared to non-tumor bearing livers. These data identified a panel of miRNAs that were highly dysregulated due to aflatoxin treatment and led to the finding that in circulation, following the dosing protocol, miR-182 was consistently increased by more than 5-fold throughout the life-course of animals subsequently diagnosed with HCC.
2. Materials & Methods
2.1. Biological Sample Selection
Tumor samples were obtained from a lifetime bioassay that has been previously described34. Male F344 rats were randomly assigned to two treatment groups: 200 μg/kg body weight (BW) AFB1 daily for four weeks, or 200 μg/kg BW AFB1 daily for four weeks plus 30 μmol/kg BW rat CDDO-Im three times per week starting one week before AFB1 dosing and continuing throughout the exposure period. All animals were maintained on a control diet following the dosing regimen34. Animals were followed during their lifetime for development of hepatocellular carcinoma. HCC diagnoses were histologically confirmed according to Eustis et al.35. All rats had multiple HCCs ranging from 13 to 20 per liver, and the time of sacrifice ranged between 79 and 95 weeks of age (Table S1). Photos of the livers that were screened for miRNAs at the termination of this study from the AFB1 and AFB1 plus CDDO-Im groups are found in Figure S1 and examples of typical histopathology of these HCCs arising in the AFB1 group are shown in Figure S2. All experiments were approved by The Johns Hopkins University Animal Care and Use Committee.
Tumors from animals previously dosed with AFB1 were selected for further analysis based on clear demarcation of a large single tumor as opposed to multiple coalesced tumors. Sections of non-tumor hepatic tissue from untreated animals and those dosed with AFB1 plus CDDO-Im were also excised. There were no tumors in any of these animals. In addition, sera from corresponding animals were analyzed from various time points of the bioassay.
2.2. RNA Isolation
2.2.1. Rat tumor & non-tumor tissue:
Total RNA was isolated from approximately 10 mg liver tissue or tumor using the miRCURY Isolation tissue kit (Exiqon, Vedbaek, Denmark) according to the manufacturer’s protocol. RNA was eluted with 50μL elution buffer (provided by manufacturer).
2.2.2. Rat sera:
Small RNAs (<1000 nts) were isolated from 50 μL serum using the miRCURY miRNA Isolation kit for biofluids (Exiqon, Vedbaek, Denmark) according to the manufacturer’s protocol. RNA was eluted with 50 μL RNase-free water (provided by manufacturer).
2.3. Small RNA Sequencing
Total RNA from tumors and non-tumor tissue samples with an RNA Integrity Number (RIN) greater than 7 identified using the 2100 Bioanalyzer (Agilent, Santa Clara, CA) were selected for sequencing. Small RNA libraries were generated using the TruSeq Small RNA Sample Preparation kit (Illumina, San Diego, CA) following the manufacturer’s protocol. Samples were prepared with a unique index and sequenced across one lane with single-end 50 bp reads. The cDNA libraries were sequenced using the HiSeq 2000 platform (Illumina, San Diego, CA).
2.4. RNA sequencing analysis
Contaminant reads with lengths less than 15 bp and a phred score less than 20 were discarded from the total output reads. The processed reads were aligned to miRNA sequences in the rat genome by conducting a BLAST search (NCBI, v.2.2.30) against a built database of rat miRNA sequences downloaded from miRBase v. 21 (www.mirbase.org)36. Hits were counted that aligned perfectly to the seed sequence, and to at least 90% of the length of the miRNA with at least 90% identity. Normalization of the count data was calculated using estimateSizeFactors. The variance estimation was calculated using estimateDispersions. Count data was averaged across all biological replicates for each treatment group and normalized to reads per million miRNA reads for each miRNA across all treatment groups (Equation 1). DESeq (Bioconductor) was used for differential expression analysis.
| (Equation 1) |
2.5. Candidate microRNA selection
Using the count data, a list was created containing the miRNAs that had greater than five-fold change between the AFB1-treated and control groups. From that list, a candidate list was generated that contained the top dysregulated miRNAs as well as functional information. A finalized panel of ten candidate miRNAs was determined that included the most dysregulated miRNAs with known biological significance from the literature.
2.6. MicroRNA expression analysis
Nine ng of eluted total RNA from tumor or tissue was reverse transcribed (RT) using miRNA specific stem-loop primers and the TaqMan miRNA Reverse Transcription Kit following the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Four μL of eluted small RNA from sera of corresponding animals was also processed in the same manner. The expression levels of candidate miRNAs were measured by real-time PCR (qPCR) using TaqMan miRNA assays (Applied Biosystems) and the StepOne Plus System (Applied Biosystems). All assays were analyzed in triplicate. The relative amount of each miRNA was calculated using the 2−ΔΔCt method37
2.7. Statistical Methods
Analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to analyze validation PCR experiments. Statistical analyses were performed using GraphPad Prism 7.0 software. Graphs indicate mean ± SD unless noted otherwise. A generalized estimating equations (GEE) model with an exchangeable correlation structure was used to analyze the longitudinal data to determine fold change with Stata 15 statistical software package. Level of significance was set at P<0.05 (*P<0.05, **P<0.005, ***P<0.001).
2.7.1. Example Stata command for GEE analysis
use “D:\GEE analysis\mmm_182full.dta”, clear xtset animal weeksofage destring tx, gen(txn) drop tx rename txn tx xtgee mir182ct tx, family(gaussian) corr(exc) eform robust
3. Results
3.1. Unique global miRNA profile in aflatoxin-induced tumors
Total RNA isolated from five hepatic tumors, each from different animals (Figure S1) initiated with AFB1, were sequenced. Liver tissues were also sequenced from five animals dosed with AFB1 plus CDDO-Im (Figure S1) as well as from three age-matched untreated animals. All of these samples were obtained from a model study that induced nearly complete incidence of HCC in rats and total HCC protection with CDDO-Im34. At the time the tissue samples used for the current study were collected, the animals had not been exposed to AFB1 for almost one year.
RNA sequencing results yielded an average of 14,132,166 clean reads for non-tumor tissues from untreated animals, 20,674,284 clean reads for tumors induced by AFB1, and 19,568,578 clean reads for non-tumor tissues from animals co-exposed to AFB1 plus CDDO-Im. The miRNA database miRBase (www.mirbase.org) was used to explore the aligned miRNA reads, revealing a total of 598 identified miRNAs. Of the identified miRNAs, 176 miRNAs were significantly expressed, as determined by abundances over 50 reads per million aligned miRNA reads. The majority of microRNAs (71%, 125 miRNAs) shared expression in the tissues of animals from all three groups (Figure 1). Twenty-two miRNAs (12.5%) were exclusively expressed in tumors and zero miRNAs were significantly co-expressed in tumors and non-tumor tissues from the AFB1 and AFB1 plus CDDO-Im treatment groups, respectively. The miRNA rno-mir-339–5p was found predominantly in non-tumor tissues from the AFB1 plus CDDO-Im group (Table 1). We found twelve miRNAs uniquely expressed in the hepatic tissue of untreated animals. Pairwise comparison analysis revealed 114 miRNAs differentially expressed in tumors and the age-matched livers of the intervention animals (Table S2).
Figure 1: Venn diagram of significantly expressed miRNAs identified by RNA-seq.
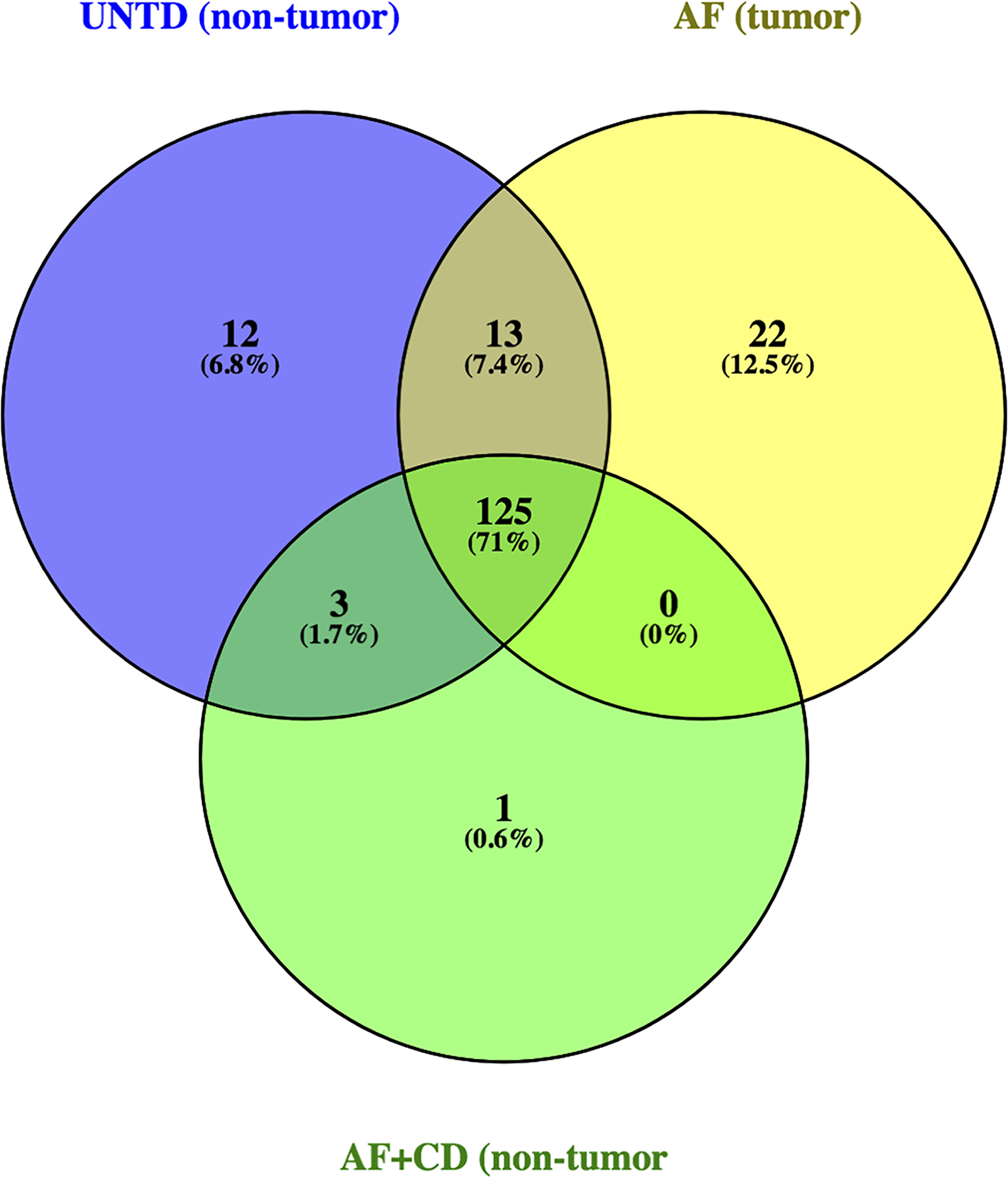
in untreated (UNTD; non-tumor), aflatoxin (AF; tumor), and aflatoxin plus CDDO-Im (AF+CD; non-tumor) groups.
Table 1:
List of exclusive and shared miRNAs by treatment group
| Group | MicroRNAs |
|---|---|
| Tumors (AF) only | 224–5p, 205, 499–5p, 132–3p, 181d-5p, 201–5p, 221–3p, 200c-3p, 96–5p, 541–5p, 27b-5p, 598–3p, 664–3p, 155–5p, 181c-3p, 652–3p, 872–3p, 3068–3p, 195–3p, 214–3p, 361–3p, 138–5p |
| Untreated only | 363–3p, 153–3p, 147, 130b-3p, 342–3p, 9a-5p, 449a-5p, 7a-5p, 139–5p, 30c-1–3p, 140–5p, 6215 |
| Untreated and Intervention (AF+CD) | 203b-3p, 144–3p, 144–5p |
| Intervention (AF+CD) | 339–5p |
Identified by RNA sequencing. Ordered by decreasing abundance.
Validation of sequencing data by RT-qPCR
To validate RNA sequencing data, a panel of miRNAs was selected for follow-up by real-time PCR analysis. Candidate miRNAs were selected by calculating ratios of miRNA count data between treatment groups, and then creating a list of top miRNAs with a dynamic range greater than a five-fold change (tumor vs. non-tumor). The following miRNAs were selected because they were most dysregulated according to these criteria: rno-miR-802–5p, 31a-5p, 224–5p, 10b-5p, 31a-5p, 205, 132–3p, 141–3p, 182, 200b-3p, and 429 (Table 2). Rno-miR-122–5p was also included because of its normal role within the liver. Of the eleven candidate miRNAs, nine were upregulated in aflatoxin-induced tumors whereas rno-miR-802–5p and 122–5p were down regulated in tumors and strongly expressed in non-tumor tissue from animals previously co-exposed to CDDO-Im.
Table 2:
List of candidate miRNAs and expression abundances by RNA sequencing
| Average count | Ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| miRNA (rno) | UNTD* | AFT† | CD‡ | Overall Ratio (UNTD:AFT:CD) | % Reduction by CDDO-Im | AFT:UNTD | CD:UNTD | AFT:CD |
| miR-10b-5p | 358 | 10797 | 290 | 1:30:1 | 97 | 30:1 | 1:1 | 37:1 |
| miR-132–3p | 24 | 119 | 4 | 1:5:0 | 96 | 5:1 | 0:1 | 27:1 |
| miR-200b-3p | 417 | 2294 | 50 | 1:6:0 | 98 | 6:1 | 0:1 | 46:1 |
| miR-205 | 12 | 203 | 2 | 1:17:0 | 99 | 17:1 | 0:1 | 92:1 |
| miR-224–5p | 1 | 388 | 1 | 1:555:1 | 100 | 555:1 | 1:1 | 388:1 |
| miR-429 | 220 | 1074 | 40 | 1:5:0 | 96 | 5:1 | 0:1 | 27:1 |
| miR-31a-5p | 906 | 4993 | 1087 | 1:6:1 | 78 | 6:1 | 1:1 | 5:1 |
| miR-141–3p | 328 | 1800 | 95 | 1:6:0 | 95 | 6:1 | 0:1 | 19:1 |
| miR-182 | 2484 | 19864 | 2933 | 1:8:1 | 85 | 8:1 | 1:1 | 7:1 |
| miR-802–5p | 895 | 172 | 1352 | 1:0:2 | −687 | 0:1 | 2:1 | 0:1 |
| miR-122–5p | 131323 | 58951 | 193087 | 1:1:2 | −286 | 1:1 | 2:1 | 0:1 |
Identified by RNA sequencing. Normalized to aligned reads per million. Percentage change by CDDO-Im compared to aflatoxin-exposed sample (AFT).
UNTD, untreated (non-tumor);
AFT, aflatoxin (tumor);
CD, aflatoxin plus CDDO-Im (non-tumor).
Figure 2 represents the candidate miRNA expression results obtained from PCR validation. In tumors, the expression of rno-miR-132–3p, 141–3p, 10b-5p, 31a-5p, 429, 182, 200b-3p, 205, and 224–5p were increased compared to non-tumor tissue from CDDO-Im intervention animals. Rno-miR-224–5p had the largest significant increase (~700 fold). The expression of these candidate miRNAs was much lower in all non-tumor tissue from animals previously dosed with AFB1 plus CDDO-Im, with the exception of rno-miR-802–5p, as seen in sequencing results.
Figure 2: Candidate miRNA expression in AFB1 tumor and AFB1 plus CDDO-Im non-tumor tissue.

Real-time PCR analysis of miRNA candidates in aflatoxin-induced tumors and non-tumor tissue from CDDO-Im intervention animals (n=10). Error bars denote mean ± SD. Dotted line at y=36 indicates limit of quantitation. Difference between treatment groups: n.s., not significant; *, p<0.05; **, p<0.005, ***, p<0.001.
3.2. Candidate miRNA levels in sera at the termination of the study
To examine whether the expression levels of candidate miRNAs measured in tumors and non-tumor tissues parallel levels in circulation, unfractionated sera from the same animals used for RNA sequencing were analyzed at the time of sacrifice by real-time PCR. All candidate miRNAs were detectable in sera. Figure 3 depicts that rno-miRs-182 and 122–5p were significantly increased in the circulation of animals diagnosed with HCC when compared to intervention animals. Increased circulating levels of rno-miR-224–5p, 802–5p, and 31a-5p were also observed in animals diagnosed with HCC, although the findings were not statistically significant.
Figure 3: Candidate miRNA expression in terminal sera.
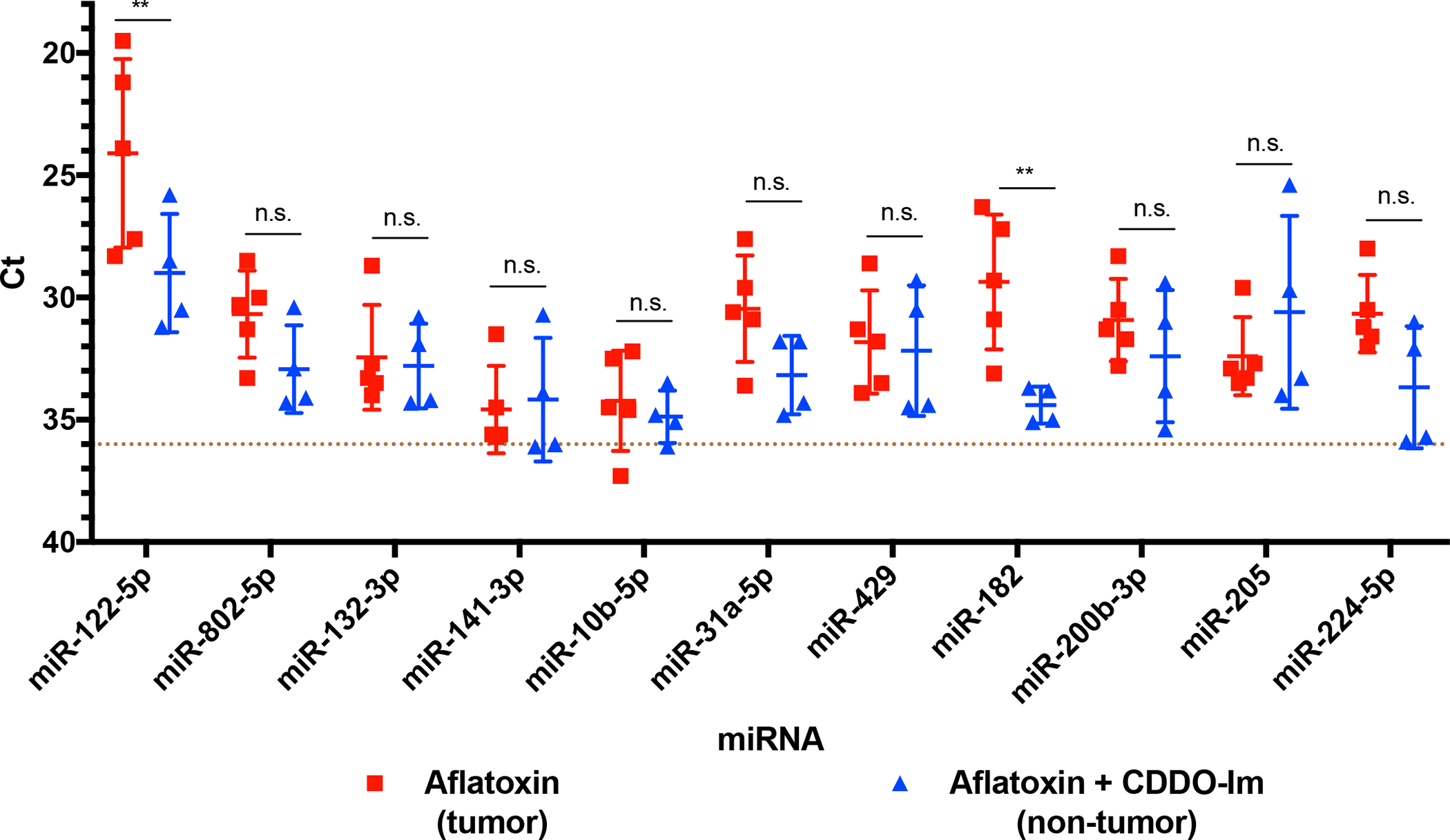
Real-time PCR analysis of miRNA candidates in sera from previously aflatoxin-exposed and aflatoxin plus CDDO-Im co-exposed animals (n=10). Error bars denote mean ± SD. Dotted line at y=36 indicates limit of quantitation. Difference between treatment groups: n.s., not significant; *, p<0.05; **, p<0.005, ***, p<0.001.
3.3. Temporal changes in circulating miRNA levels
The samples analyzed and shown in Figure 3 were obtained at the most advanced stages of HCC (shown in AFB1 dosed animals in Figure S1) when necrosis and other biological processes occurring may skew the alteration of serum miRNA levels. Since longitudinal serum samples had been collected at monthly intervals, miRNA levels in the circulation of animals across the trajectory to disease could be explored. This would allow identifying the emergence of miRNAs that may be attributable to the disease process and therefore be predictive. The sera obtained starting at 17 weeks of age and continuing monthly until sacrifice were analyzed by qPCR. It is also important to note that aflatoxin-treated animals began to develop palpable tumors around 70 weeks of age, which was observed during blood collection. For many of the candidate miRNAs, the circulating levels were similar between treatment groups with fold changes less than 2 (e.g. rno-miR-224–5p, 10b-5p, 429, 200b-3p) (Figure 4). In Figure 4 are shown miR-122–5p and miR-802–5p, and miR-182 displayed in Figure 5 revealed statistically significant differences in circulating levels between treatment groups, with fold changes of 4.66 (95% CI: 3.49–6.23), 2.60 (95% CI: 1.82–3.71), and 5.13-fold change (95% CI: 4.59–5.70), respectively. Serum rno-miR-182 was the only miRNA that tracked with a significant difference in levels between the aflatoxin and aflatoxin plus CDDO-Im dosed animals at every time point (Figure 5). This difference was further amplified when measuring rno-miR-182 levels in terminal sera (filled symbols).
Figure 4: Longitudinal analysis of candidate miRNAs in serum.
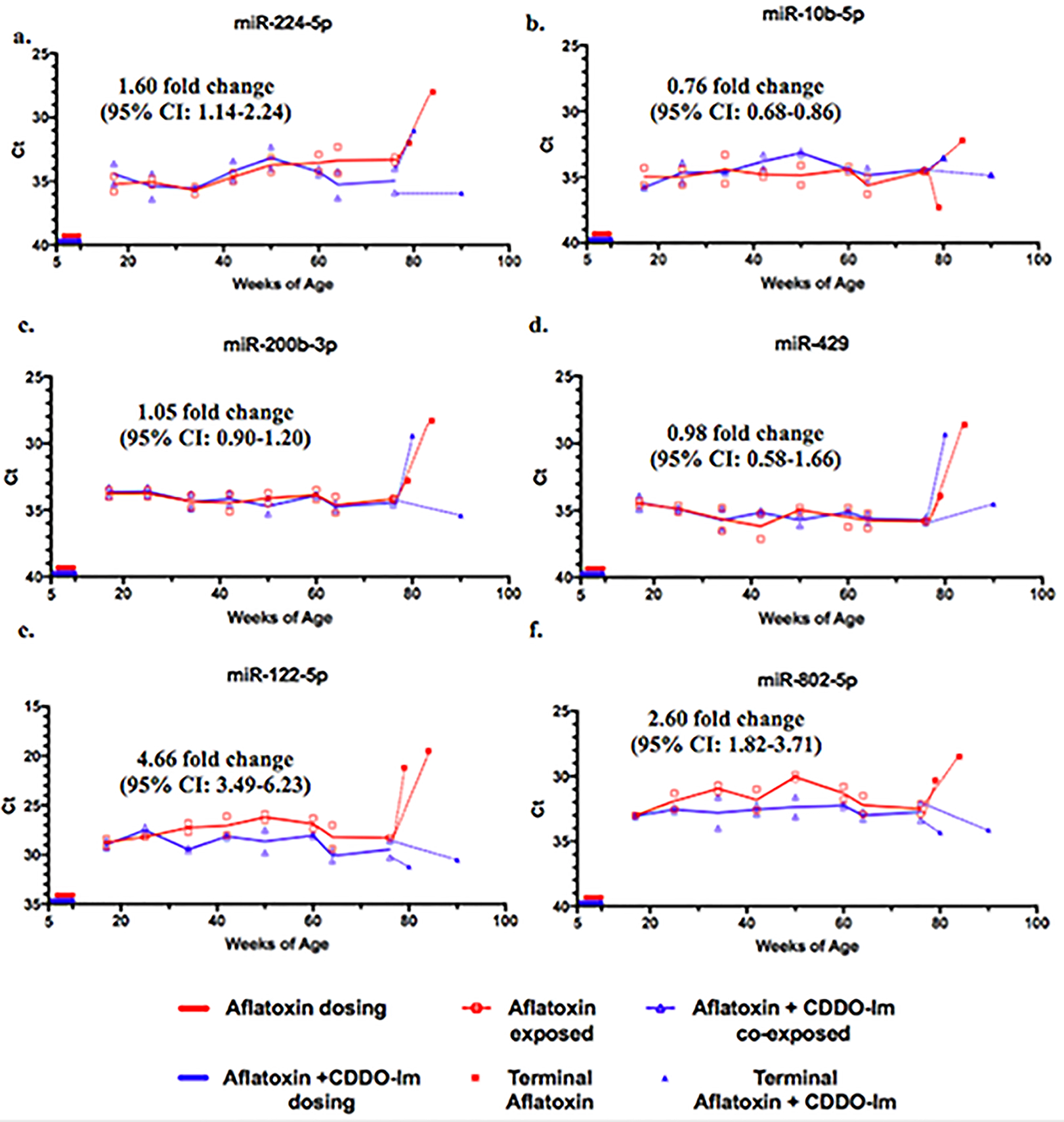
Repetitive measurements of candidate miRNAs from the sera of previously aflatoxin-exposed and aflatoxin plus CDDO-Im co-exposed animals by real-time PCR (n=10). Open symbols indicate individual replicates with means connected. Closed symbols indicate miRNA levels at time of sacrifice.
Figure 5: Longitudinal analysis of serum miR-182.

Repetitive measurements of serum rno-miR-182 from aflatoxin-exposed and aflatoxin plus CDDO-Im co-exposed animals by real-time PCR (n=10). Open symbols indicate individual replicates with means connected. Closed symbols indicate miRNA levels at time of sacrifice. Dotted line at y=36 indicates limit of quantitation.
4. Discussion
In this study, analysis of a unique sample set allowed for the identification of miRNAs dysregulated in HCC progression. This sample set was obtained from a quantitative rat cancer model where the individualized outcomes were known for each animal following their initiation of carcinogenesis during a 28-day dosing regimen34. Of the animals selected for further analysis in this study, those dosed with AFB1 began to develop palpable tumors and concomitant weight loss at 70 or more weeks of age and were formally diagnosed with HCC at the time of sacrifice (Figure S1). Animals in the AFB1 plus CDDO-Im group did not develop HCC (Figure S1). Transcriptomic analysis of tumors from aflatoxin-treated animals and non-tumor tissues from animals co-exposed to the chemopreventive agent CDDO-Im exhibited a perturbed global miRNA profile in tumors. Specifically, rno-miRs-224–5p, 205, 200b-3p, 182, 429, 31a-5p, 10b-5p, 141–3p, 132–3p, and 802–5p were found to be the most dysregulated. The sequences were also verified for homology to the respective human miRs. MiR-224–5p is noted in the literature to be commonly upregulated in HCC, can promote tumor cell migration and invasion, and targets cell cycle checkpoints such as, p2126,38. Elevated serum levels of this miR have also been observed in early stages of HCC26. Rno-miR-31a-5p is increased in plasma after liver injury and differs from its human homolog hsa-miR-31 by one nucleotide36,39,40. In humans, miR-31 is associated with aging livers and is increased in tumors, correlating with cirrhosis28,41,42. Other miRNAs found upregulated in tumors, such as the miR-200 family (e.g. rno-miRs-200b-3p, 429, 141–3p) and rno-miR-205 are known to play a role in angiogenesis and epithelial to mesenchymal transition in multiple cancers including HCC43,44. MiR-802–5p was the only candidate selected that was significantly decreased in tumors. This miRNA is noted to play a tumor suppressive role in different cancers and demonstrates moderate tumor suppressive activity against c-Myc driven hepatocarcinogenesis in mice45. In terminal sera, however, rno-miR-802–5p was elevated in animals with HCC. This result supports a study by Wolenski et al. that points to this miRNA as a plasma marker of liver injury39.
Levels of serum miRs-182 and 122–5p were also elevated in animals diagnosed with HCC. Longitudinal analysis of circulating miRNAs revealed rno-miR-182 as a potential predictive biomarker, which is 96% identical in sequence to its human homolog. MiR-182 is another oncomiR that is commonly upregulated in multiple cancers and predicts poor survival in patients46–50. Chen et al. observed that high levels of serum miR-182 in HCC patients were significantly associated with decreased survival after surgery22. Functionally, this miRNA targets tumor suppressor genes CCAAT enhancer binding protein (CEBPA) and forkhead box O 1 (FOXO1)48,51. It has been also shown to affect WNT/β-catenin and AKT signaling by targeting forkhead box O 3a (FOXO3a) in tumor tissue which resulted in HCC proliferation52. Within this study, increased miR-182 could be altering these signaling pathways and increasing tumor cell proliferation. While we did not investigate the origin of serum candidate miRNAs, miR-182 could be acting after release from cancer cells or from cells reprogrammed due to injury by AFB1 (e.g., encapsulated in exosomes). We did not use imaging to track the development of hepatic tumors; however, they did not become palpable until about 70 weeks of age in the AFB1-treated rats. Given the detection of miR-182 in sera at 17 weeks, it is likely its elevated expression initially arises from pre-neoplastic tissue earlier in the carcinogenic process. Significant spikes in serum miR-182 were observed between the last scheduled monthly sample to the terminal sample in 4 out of 5 tumor-bearing rats, suggesting that tumors are nonetheless a substantial source. This aspect should be investigated in future experiments. The results from the present study show significant elevation of this miRNA in sera observed less than two months after the AFB1 dosing period and considerably before diagnosis (sacrifice). It is remarkable that this increase is maintained throughout the lifetimes of the animals during such a complex and multifactorial disease like cancer. To our knowledge, this is the first study to indicate a miRNA that is truly predictive for risk stratification of hepatocellular carcinoma.
In summary, the results presented here describe a panel of miRNAs highly dysregulated in aflatoxin-induced hepatic tumors. This dysregulation translates to sera for miRs-122–5p and 182, with the latter displaying increased expression throughout development of disease. Further validation of this finding is needed in human samples. For example, future experiments could measure serum miR-182 levels in random samples from a prospective human HCC study. In addition, serum miRs-122–5p and 802–5p could be explored as potential biomarkers as these also were significantly elevated throughout the lifetimes of animals with HCC. Validation of serum miR-182 can lead to alternative screening and surveillance methods for individuals with high risk of developing HCC.
Supplementary Material
Figure S1. Photos of each of the five livers from the aflatoxin-only treated group and five livers from the aflatoxin plus CDDO-Im treated group. These photos illustrate the gross anatomy of the livers that were used in this investigation to isolate miRNAs from excised tumors and age matched chemoprotected animals.
Figure S2. Examples of typical histopathology at 63X magnification of HCC developing in AFB1-treated rats from which tumor RNA samples were obtained.
Table S1. Selection of tumors from each animal used in this study
Table S2. Complete set of miRNAs detected in the control, aflatoxin-only and aflatoxin plus CDDO-Im rat liver tissues. The counts shown are an average of the individual animal samples.
Acknowledgements
We thank the Next Generation Sequencing Center (Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine) for assistance with RNA sequencing and data analysis.
Funding Support:
This research supported by NIH grants T32 ES007141, P30 CA006973, and R35 CA197222.
Abbreviations:
- AFB1
Aflatoxin B1
- CDDO-Im
1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole
- miRNA or miR
microRNA
- HCC
hepatocellular carcinoma
Footnotes
Data Availability Statement:
Data on the miRNA analysis will be available on request to the corresponding author.
References
- 1.Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2013;2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 390(10100):1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 3.Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: Where are we? World Journal of Experimental Medicine. 2016;6(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53(2):291–297. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World journal of gastroenterology : WJG. 2015;21(37):10573–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. [DOI] [PubMed] [Google Scholar]
- 7.Shah S, Bellows BA, Adedipe AA, Totten JE, Backlund BH, Sajed D. Perceived barriers in the use of ultrasound in developing countries. Critical Ultrasound Journal. 2015;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahsani Z, Mohammadi-Yeganeh S, Kia V, Karimkhanloo H, Zarghami N, Paryan M. WNT1 Gene from WNT Signaling Pathway Is a Direct Target of miR-122 in Hepatocellular Carcinoma. Applied biochemistry and biotechnology. 2017;181(3):884–897. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed AA, Ali-Eldin ZA, Elbedewy TA, El-Serafy M, Ali-Eldin FA, AbdelAziz H. MicroRNAs and clinical implications in hepatocellular carcinoma. World journal of hepatology. 2017;9(23):1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XY, Feng XZ, Tang JZ, et al. MicroRNA-200b inhibits the proliferation of hepatocellular carcinoma by targeting DNA methyltransferase 3a. Mol Med Rep. 2016. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Di C, Li W, et al. Oncomirs miRNA-221/222 and Tumor Suppressors miRNA-199a/195 Are Crucial miRNAs in Liver Cancer: A Systematic Analysis. Digestive Diseases and Sciences. 2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang LK, Yang YT, Ma X, et al. MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell death & disease. 2016;7:e2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Rie D, Abugessaisa I, Alam T, et al. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nature biotechnology. 2017;35(9):872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Zhang R, Shen Y, Liu G, Lu X, Wu CI. The evolution of evolvability in microRNA target sites in vertebrates. Genome research. 2013;23(11):1810–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Current opinion in lipidology. 2012;23(2):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9(6):654–U672. [DOI] [PubMed] [Google Scholar]
- 17.Okajima W, Komatsu S, Ichikawa D, et al. Circulating microRNA profiles in plasma: identification of miR-224 as a novel diagnostic biomarker in hepatocellular carcinoma independent of hepatic function. Oncotarget. 2016;7(33):53820–53836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Q, Gong L, Wang J, et al. miR-10b exerts oncogenic activity in human hepatocellular carcinoma cells by targeting expression of CUB and sushi multiple domains 1 (CSMD1). BMC Cancer. 2016;16(1):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Hou P, Wu Z, Wang T, Nie Y. Circulating miR-375 and miR-199a-3p as potential biomarkers for the diagnosis of hepatocellular carcinoma. Tumor Biology. 2015;36(6):4501–4507. [DOI] [PubMed] [Google Scholar]
- 20.Niu JX, Meng XK, Ren JJ. Studied microRNA gene expression in human hepatocellular carcinoma by microRNA microarray techniques. World journal of gastroenterology : WJG. 2015;21(44):12605–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang L, Cheng Q, Zhang BH, Zhang MZ. Circulating microRNAs as biomarkers in hepatocellular carcinoma screening: a validation set from China. Medicine. 2015;94(10):e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Chu F, Cao Y, Shao J, Wang F. Serum miR-182 and miR-331–3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol. 2015;36(10):7439–7447. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of Serum Exosomal MicroRNA-21 in Human Hepatocellular Carcinoma. BioMed Research International. 2014;2014:864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu AM, Yao TJ, Wang W, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ open. 2012;2(2):e000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Kosaka N, Tanaka M, et al. MicroRNA-500 as a potential diagnostic marker for hepatocellular carcinoma. Biomarkers. 2009;14(7):529–538. [DOI] [PubMed] [Google Scholar]
- 26.Lin L, Lu B, Yu J, Liu W, Zhou A. Serum miR-224 as a biomarker for detection of hepatocellular carcinoma at early stage. Clin Res Hepatol Gastroenterol. 2016;40(4):397–404. [DOI] [PubMed] [Google Scholar]
- 27.Gougelet A, Sartor C, Bachelot L, et al. Antitumour activity of an inhibitor of miR-34a in liver cancer with beta-catenin-mutations. Gut. 2015. [DOI] [PubMed] [Google Scholar]
- 28.Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52(4):297–303. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Yu H, Zhang Y, et al. Upregulation of miR-34a-5p antagonizes AFB1-induced genotoxicity in F344 rat liver. Toxicon. 2015;106:46–56. [DOI] [PubMed] [Google Scholar]
- 30.Yang W, Lian J, Feng Y, et al. Genome-wide miRNA-profiling of aflatoxin B1-induced hepatic injury using deep sequencing. Toxicology Letters. 2014;226(2):140–149. [DOI] [PubMed] [Google Scholar]
- 31.Livingstone MC, Johnson NM, Roebuck BD, Kensler TW, Groopman JD. Profound changes in miRNA expression during cancer initiation by aflatoxin B1 and their abrogation by the chemopreventive triterpenoid CDDO-Im. Mol Carcinog. 2017;56(11):2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6(1):154–162. [DOI] [PubMed] [Google Scholar]
- 33.Cuadrado A, Rojo AI, Wells G, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 2019. [DOI] [PubMed] [Google Scholar]
- 34.Johnson NM, Egner PA, Baxter VK, et al. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev Res (Phila). 2014;7(7):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen X, Donepudi AC, Thomas PE, Slitt AL, King RS, Aleksunes LM. Regulation of Hepatic Phase II Metabolism in Pregnant Mice. The Journal of Pharmacology and Experimental Therapeutics. 2013;344(1):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 2014;42(D1):D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 38.An F, Olaru AV, Mezey E, et al. MicroRNA-224 Induces G1/S Checkpoint Release in Liver Cancer. Journal of clinical medicine. 2015;4(9):1713–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolenski FS, Shah P, Sano T, et al. Identification of microRNA biomarker candidates in urine and plasma from rats with kidney or liver damage. Journal of applied toxicology : JAT 2017;37(3):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith A, Calley J, Mathur S, et al. The Rat microRNA body atlas; Evaluation of the microRNA content of rat organs through deep sequencing and characterization of pancreas enriched miRNAs as biomarkers of pancreatic toxicity in the rat and dog. BMC genomics. 2016;17:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capri M, Olivieri F, Lanzarini C, et al. Identification of miR-31–5p, miR-141–3p, miR-200c-3p, and GLT1 as human liver aging markers sensitive to donor-recipient age-mismatch in transplants. Aging cell. 2017;16(2):262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojcicka A, Swierniak M, Kornasiewicz O, et al. Next generation sequencing reveals microRNA isoforms in liver cirrhosis and hepatocellular carcinoma. The international journal of biochemistry & cell biology. 2014;53:208–217. [DOI] [PubMed] [Google Scholar]
- 43.Dhayat SA, Mardin WA, Kohler G, et al. The microRNA-200 family--a potential diagnostic marker in hepatocellular carcinoma? J Surg Oncol. 2014;110(4):430–438. [DOI] [PubMed] [Google Scholar]
- 44.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. [DOI] [PubMed] [Google Scholar]
- 45.Tao J, Ji J, Li X, et al. Distinct anti-oncogenic effect of various microRNAs in different mouse models of liver cancer. Oncotarget. 2015;6(9):6977–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia L, Luo S, Ren X, et al. miR-182 and miR-135b Mediate the Tumorigenesis and Invasiveness of Colorectal Cancer Cells via Targeting ST6GALNAC2 and PI3K/AKT Pathway. Dig Dis Sci. 2017. [DOI] [PubMed] [Google Scholar]
- 47.Visani M, de Biase D, Marucci G, et al. Expression of 19 microRNAs in glioblastoma and comparison with other brain neoplasia of grades I-III. Molecular oncology. 2014;8(2):417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Ren R, Hu H, et al. MiR-182 is up-regulated and targeting Cebpa in hepatocellular carcinoma. Chin J Cancer Res. 2014;26(1):17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang QH, Sun HM, Zheng RZ, et al. Meta-analysis of microRNA-183 family expression in human cancer studies comparing cancer tissues with noncancerous tissues. Gene. 2013;527(1):26–32. [DOI] [PubMed] [Google Scholar]
- 50.Wang F, Zhong S, Zhang H, et al. Prognostic Value of MicroRNA-182 in Cancers: A Meta-Analysis. Disease markers. 2015;2015:482146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weidinger C, Krause K, Klagge A, Karger S, Fuhrer D. Forkhead box-O transcription factor: critical conductors of cancer’s fate. Endocrine-related cancer. 2008;15(4):917–929. [DOI] [PubMed] [Google Scholar]
- 52.Cao MQ, You AB, Zhu XD, et al. miR-182–5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J Hematol Oncol. 2018;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Photos of each of the five livers from the aflatoxin-only treated group and five livers from the aflatoxin plus CDDO-Im treated group. These photos illustrate the gross anatomy of the livers that were used in this investigation to isolate miRNAs from excised tumors and age matched chemoprotected animals.
Figure S2. Examples of typical histopathology at 63X magnification of HCC developing in AFB1-treated rats from which tumor RNA samples were obtained.
Table S1. Selection of tumors from each animal used in this study
Table S2. Complete set of miRNAs detected in the control, aflatoxin-only and aflatoxin plus CDDO-Im rat liver tissues. The counts shown are an average of the individual animal samples.


