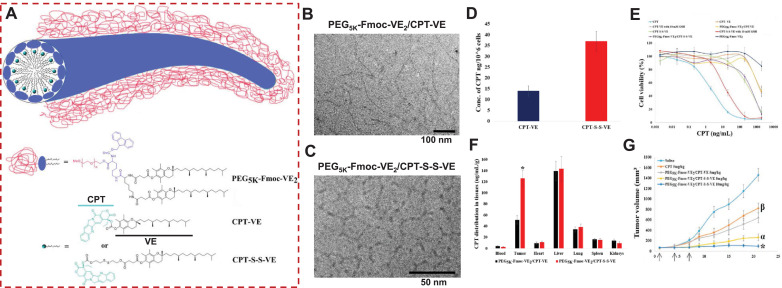
Fig. 4.
(A) Mechanistic representation of self-assembly of CPT-VE or CPT-S-S-VE to nanofibers upon stabilization by PEG5K-Fmoc-VE2. Cryo-electron microscopy (cryoEM) imaging of PEG5K-Fmoc-VE2/CPT-S-S-VE (B) and PEG5K-Fmoc-VE2/CPT-VE (C). (D) Intracellular release of CPT in 4T1.2 cells treated with CPT-VE and CPT-S-S-VE (100 ng/mL in terms of CPT) for 24 h. (E) The cytotoxicity of different CPT formulations in 4T1.2 cancer cells treated with indicated concentrations for 72 h and was then assessed by MTT assay. (F) Tissue biodistribution of PEG5K-Fmoc-VE2/CPT-VE and PEG5K-Fmoc-VE2/CPT-S-S-VE (5 mg CPT/kg) in 4T1.2-tumor bearing mice. *p < 0.001, compared to PEG5K-Fmoc-VE2/CPT-VE. (G) In vivo therapeutic efficacy of various VE-derived CPT prodrug nanotherapeutics in breast tumor mouse model (n = 5). Arrows stand for the IV injection. *p < 0.01, compared to PEG5K-Fmoc-VE2/CPT-S-S-VE (5 mg/kg); αp < 0.001, compared to PEG5K-Fmoc-VE2/CPT-VE (5 mg/kg) and CPT (5 mg/kg); βp < 0.001, compared to saline. Reproduced with permission from [9] . (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)